Invasive Nontyphoidal Salmonellosis: An Antimicrobial-Resistant Foe
Salmonella becomes more dangerous as antimicrobial resistance grows. Read about its most common manifestations.
Salmonella enterica subspecies enterica is a motile, Gram-negative bacteria divided into typhoidal serotypes (Salmonella Typhi and Paratyphi) and nontyphoidal serotypes (NTS). Although there are more than 2500 known NTS, approximately 100 are reported to cause human disease.1 T he primary serotypes isolated in the United States are Enteritidis (20%), Typhimurium (16%), Newport (11%), I4, [5], 12:i:- (4%), and Heidelberg (4%).2
The global burden of typhoidal Salmonella is high, causing approximately 22 million infections and more than 200,000 deaths annually. However, the impact within the United States is limited, causing only approximately 400 infections and one death in 2016.3 NTS are estimated to cause 1.35 million annual cases in the United States, predominantly manifesting isolated, self-limited gastrointestinal illness; however, a subset progresses to severe invasive disease.4
The global burden of invasive nontyphoidal salmonellosis (iNTS) was estimated to be 535,000 cases, resulting in 77,500 deaths in 2017.5 Transmission can occur through foodborne exposures or direct animal contact, but human-to-human transmission is rare.1 iNTS can occasionally manifest as serious, life-threatening illnesses requiring antimicrobial intervention.1,4 Over the past several decades, the rates of antimicrobial-resistant NTS have continued to increase as agricultural antibiotic use increases,6 subsequently limiting empiric antimicrobial choices in serious illness.
MANIFESTATIONS AND DIAGNOSIS
The primary manifestation of NTS is gastroenteritis, which can be more severe than other types of infectious gastroenteritis.7 In some cases, Salmonella can cause systemic dissemination, resulting in bacteremia and metastatic foci of infection, including meningitis, arteritis, and osteomyelitis.1 The CDC estimates approximately 8% of NTS are invasive in the United States, which is similar to a global estimate of 6%, although this likely represents an underestimation because of inadequate sampling.4,5 Host factors and pathogen virulence can determine risk for translocation from the enteric system.
Patients with diminished cell-mediated immunity are at higher risk of iNTS, including those with HIV or malignancies and those who are on glucocorticoids or immunosuppression.5 There may be an association between multidrug resistance and invasive infection, as one study found that NTS with multidrug resistance were more likely to cause bacteremia.8 It is unclear why there was an association between multidrug resistance and invasive infection. In patients without recent gastroenteritis presenting with NTS bacteremia, metastatic foci of infection should be investigated.
Endovascular foci of infection are a rare but serious complication of iNTS and are associated with a mortality of more than 50%.1 These infections can manifest as endocarditis or as mycotic aneurysms, which are more common in patients with a history of atherosclerosis. Endocarditis is frequently destructive and can be evaluated with an echocardiogram. Evaluating for mycotic aneurysm may be more challenging, as imaging alone may not necessarily be diagnostic of active infection; however, the presence of aneurysm and bacteremia is suggestive. Patients with gas or inflammation surrounding a previously known aneurysms in the setting of bacteremia should be suspected to be infection. A PET scan may also be useful in localizing infection.1 The primary mechanism of infection of the aorta is via hematogenous seeding, and blood cultures are positive in more than 50% of cases. However, contiguous spread of infection from vertebral osteomyelitis can also occur.1
In adults, vertebral osteomyelitis is the most common osteoarticular manifestation. However, there are rare reports of prosthetic joint infections, as well. Cultures should be obtained from suspected sites of infection in bone and joint infections.1 Meningitis is a rare manifestation of iNTS. Most infections occur in infants and neonates or those with other immunocompromising conditions. Diagnosis occurs with obtaining a cerebrospinal f luid sample and culture.9
ANTIMICROBIAL RESISTANCE
Antimicrobial resistance in NTS is not uncommon. One study reported 12% of isolates demonstrating resistance to at least 1 antibiotic (ampicillin, ceftriaxone, or ciprofloxacin) between 2004 and 2012. Resistance to ampicillin was most common (6.5%), whereas ceftriaxone resistance was detected in 3.5% of isolates, and ciprofloxacin nonsusceptibility was isolated in 2.4%. Only 0.2% of isolates were nonsusceptible to all 3 antibiotics.
In contrast, of isolates reported to the National Antimicrobial Resistance Monitoring System between 2016 and 2018, 8.7% were resistant to ampicillin, 3.2% were resistant to both ceftriaxone and ampicillin, and 7.1% were nonsusceptible to ciprofloxacin. The percentage of isolates resistant to all 3 remained low, at 1.1%(FIGURE 1).10
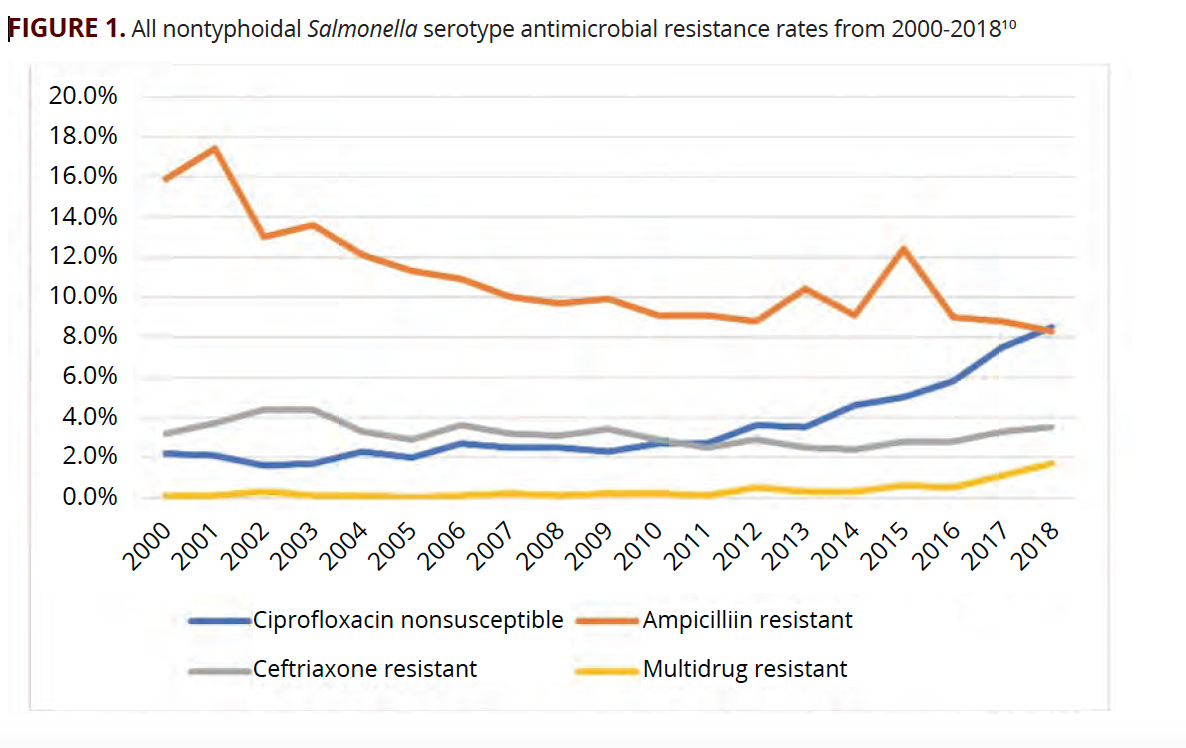
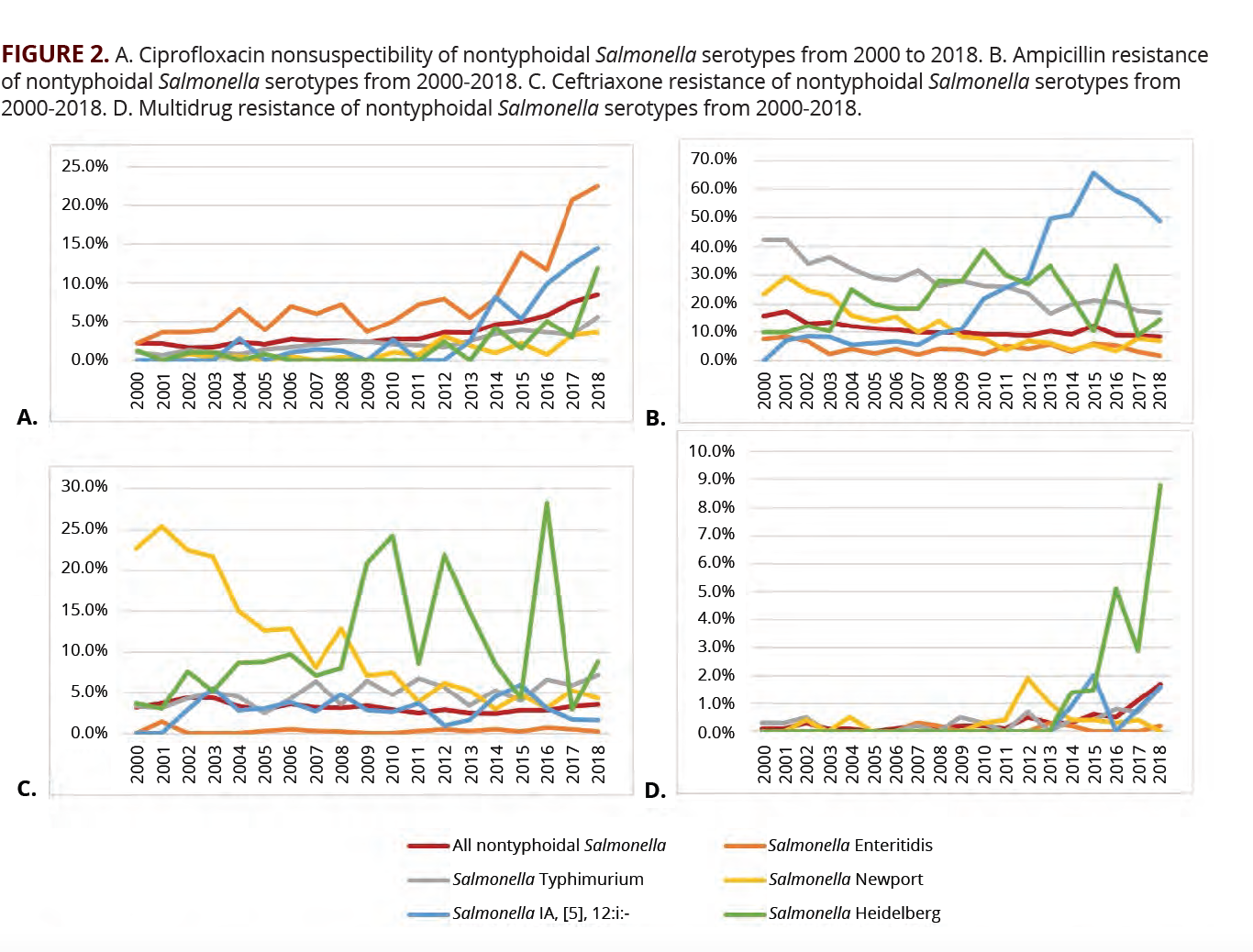
The evolution of antimicrobial resistance in NTS has varied by serotype. Enteritidis had the largest increase in resistance to ciprofloxacin. In 2000, 2.2% of isolates were nonsusceptible to ciprofloxacin vs 22.5% in 2018 (FIGURE 2A). All other serotypes have had increased ciprofloxacin nonsusceptibility over the same time frame (FIGURE 2A). Trends of ceftriaxone resistance have been more variable (FIGURE 2B). Overall, ampicillin resistance has trended down but remains high (FIGURE 2C). The percentage of multidrug resistant isolates of all serotypes remains low but has been increasing in recent years, with Salmonella Heidelberg having the highest rate of multidrug resistance (FIGURE 2D). Once identification of serotype has been obtained, this can be used to predict sensitivity to antibiotics.10
NTS are associated with foodborne illness and animal exposure, and it is intuitive that part of the reason antimicrobial resistance is increasing in this organism is associated with the use of antimicrobials in the animal husbandry population. Consequently, as the use of cephalosporins has been decreasing as of 2012, isolation of cephalosporin-resistant NTS in animals (while still significantly higher than found in humans) has also decreased.6 This may account for the decrease in ceftriaxone resistance that has been observed in human clinical isolates. Although fluoroquinolone use was discontinued in poultry in 2005, it is still widely used in cattle to treat respiratory infections.6 It is difficult to determine whether the relationship between the use of fluoroquinolone in agriculture is associated with the increase in fluoroquinolone nonsusceptibility in humans, as fluoroquinolone nonsusceptibility is not frequently assessed in animal husbandry populations.6
TREATMENT
In patients with disease limited to the gastrointestinal tract, routine administration of antibiotics is not recommended, as it does not decrease the length of diarrheal illness and increases the risk of progression to asymptomatic carriage of NTS.11 Antimicrobial therapy should be considered in patients whose progression to invasive disease is more likely, including neonates, patients with HIV, patients older than 50 with atherosclerosis, cardiovascular disease, significant joint pathology, and those on immunosuppression.7
Patients who progress to iNTS should be treated with antibiotics. The recommended empiric antibiotic treatment has evolved over the past decade, as antimicrobial resistance has increased. Although no formal recommendations exist, combination therapy with ceftriaxone and fluoroquinolone is reasonable in severe infection until susceptibilities are confirmed to determine which agent will be effective, as multidrug resistance is rare.12 Ampicillin, ceftriaxone, and fluoroquinolones remain cornerstones of treatment when susceptibilities are known.
Patients with mycotic aneurysms should be treated with effective antibiotics and assessed for surgical intervention. A study by Soravia-Dunand et al showed that mortality of mycotic aneurysm treated with medical management alone was 96% compared with 40% with combined surgical and medical management.13 Previously, patients with Salmonella mycotic aneurysms were treated with open repair, but endovascular aneurysm repair (EVAR) has been used more recently with some success.1 A meta-analysis evaluating all causes of mycotic aortic aneurysms did not find evidence to support either approach and recommend evaluating individuals on a case-by-case basis. Antibiotic pretreatment was associated with improved outcomes regardless of approach. Consideration for long-term suppression must also be made after EVAR, as there is prosthetic material implanted directly into an infected field.14
Early osteomyelitis can be cured with antimicrobial therapy alone. In a meta-analysis, 45% of patients reviewed were treated with antimicrobial therapy alone, of which 80% were successfully cured. Of the patients treated with a combination of surgical intervention and antimicrobial therapy, 75% were cured. The mean duration of antimicrobial therapy was 11 weeks.15 Limited data on prosthetic joint infection and septic arthritis secondary to Salmonella exists. These infections are managed with a combination of antimicrobial therapy and surgical intervention. Best practices in prosthetic joint infection management frequently include 2-stage device revision; however, this management should be performed in consultation with appropriate surgical teams.16
Most literature regarding antibiotic choice and duration of therapy for Salmonella meningitis has been in pediatric populations, as central nervous system involvement most frequently manifests in infants and neonates. The American Academy of Pediatrics recommends a third-generation cephalosporin for a duration of 4 to 6 weeks due to high rates of treatment failure. Repeating lumbar punctures may be indicated after 48 hours to ensure clearance and, if persistently positive, consideration of dual therapy with a fluoroquinolone should be pursued.17
CONCLUSIONS
Nontyphoidal Salmonella infections have a wide array of clinical presentations, including gastroenteritis requiring no antimicrobial intervention, invasive NTS disease necessitating systemic antibiotics at a minimum, and in some cases, requiring surgical debridement and possible lifelong suppressive therapy. Increasing antimicrobial resistance of NTS has complicated empiric antimicrobial choice while awaiting isolation and susceptibility. Antibiotic overuse in the treatment of human disease and in agriculture has contributed to the continued escalation of antimicrobial resistance in NTS. Ongoing antimicrobial stewardship practices in both populations can help slow the trajectory of antibiotic resistance, thereby improving empiric options available for treatment of invasive nontyphoidal Salmonella infections.
References
1.Fierer J. Invasive non-typhoidal Salmonella (iNTS) infections. Clin Infect Dis. 2022;ciac035. doi:10.1093/cid/ciac035
2.Medalla F, Gu W, Mahon BE, et al. Estimated Incidence of antimicrobial drug-resistant nontyphoidal Salmonella infections, United States, 2004-2012. Emerg Infect Dis. 2016;23(1):29-37. doi:10.3201/eid2301.160771
3.National Typhoid and Paratyphoid Fever Surveillance Annual Summary, 2016. CDC. Updated September 14, 2021. Accessed July 20, 2022. https://www.cdc.gov/typhoid-fever/reports/annual-report-2016.html
4.Information for healthcare professionals and laboratories. CDC. Updated July 18, 2022. Accessed July 20, 2022. https://www.cdc.gov/salmonella/general/technical.html
5.GBD 2017 Non-Typhoidal Salmonella Invasiv Disease Collaborators. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19(12):1312-1324. doi:10.1016/S1473-3099(19)30418-9
6.McDermott PF, Zhao S, Tate H. Antimicrobial resistance in nontyphoidal Salmonella. Microbiol Spectr. 2018;6(4): 10.1128/microbiolspec.ARBA-0014-2017. doi:10.1128/microbiolspec.ARBA-0014-2017
7.Shane AL, Mody RK, Crump JA, et al. 2017 Infectious Diseases Society of America Clinical Practice Guidelines for the Diagnosis and Management of Infectious Diarrhea. Clin Infect Dis. 2017;65(12):1963-1973. doi:10.1093/cid/cix959
8.Akullian A, Montgomery JM, John-Stewart G, et al. Multi-drug resistant non-typhoidal Salmonella associated with invasive disease in western Kenya. PLoS Negl Trop Dis. 2018;12(1):e0006156. doi:10.1371/journal.pntd.0006156
9.Keddy KH, Sooka A, Musekiwa A, et al. Clinical and microbiological features of Salmonella meningitis in a South African population, 2003-2013. Clin Infect Dis. 2015;61(suppl 4):S272-S282. doi:10.1093/cid/civ685
10.NARMS Now: Human Data. CDC. Accessed July 20, 2022. https://wwwn.cdc.gov/narmsnow/
11.Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012;11(11):CD001167. doi:10.1002/14651858.CD001167.pub2
12.Pegues DA, Miller SI. Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Ninth ed. Elsevier; 2020:2725-2736.
13.Soravia-Dunand VA, Loo VG, Salit IE. Aortitis due to Salmonella: report of 10 cases and comprehensive review of the literature. Clin Infect Dis. 1999;29(4):862-868. doi:10.1086/520450
14.Sörelius K, Budtz-Lilly J, Mani K, Wanhainen A. Systematic review of the management of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg. 2019;58(3):426-435. doi:10.1016/j.ejvs.2019.05.004
15.Huang ZD, Wang CX, Shi TB, et al. Salmonella osteomyelitis in adults: a systematic review. Orthop Surg. 2021;13(4):1135-1140. doi:10.1111/os.12912
16.Sebastian S, Dhawan B, Malhotra R, Gautam D, Kapil A. Salmonella typhimurium infection in total knee arthroplasty: a case report with review of literature. J Lab Physicians. 2017;9(3):217-219. doi:10.4103/0974-2727.208254
17.Wen SC, Best E, Nourse C. Non-typhoidal Salmonella infections in children: review of literature and recommendations for management. J Paediatr Child Health. 2017;53(10):936-941. doi:10.1111/jpc.13585

Newsletter
Stay ahead of emerging infectious disease threats with expert insights and breaking research. Subscribe now to get updates delivered straight to your inbox.




















