BOSTON — Rachel Haurwitz, PhD, greets me with a congenial smile as she sits in the press room at the Boston Convention Center, one of 14,000 attendees at the BIO 2023 conference. Her coolness and precision, both temporally and conversationally, are a stark contrast to my fumbling with belongings as I wipe the sweat off my forehead, straighten my clothes, and take a tardy seat to kick off my second day at the conference.
A decade ago, Haurwitz jumped from being a humble graduate student in Jennifer Doudna’s lab to launching a biotech start-up as co-founder and chief executive. “I went straight from graduate school to being the founding CEO of this company,” says Haurwitz. “This is the only job I’ve ever had!”

Haurwitz walks me through some of the first stops that Caribou Biosciences made on its twelve-year journey taking CRISPR gene editing technology into the clinic. There’s some work with Novartis on high-throughput target identification and validation while simultaneously doing industrial microbe engineering with Dupont.
“Caribou 1.0 was a pure technology platform company,” says Haurwitz conscientiously. “We explicitly did not develop our own products, instead we partnered with other companies to help them deploy genome editing in research or product development. But Caribou today looks very different from Caribou in the early days.”
In an effort to refocus Caribou, Haurwitz took a step back and began to survey the competition. She saw that there was a bottleneck with the specificity of genome editing—actually editing the target site while avoiding potentially deleterious off-target effects. Haurwitz chuckles: Easy to say, hard to do.
This reconnaissance helped Haurwitz and her team focus years of research into inventing the next generation of CRISPR technology based on hybrid RNA-DNA guides—chRDNAs (pronounced “chardonnays”)—that direct precise genome editing. Caribou is deploying the Cas12a chRDNA technology to carry out high-efficiency multiple edits, including multiplex gene insertions, to develop CRISPR-edited therapies.
“It’s far more specific than the first-generation [CRISPR technologies] and really allowed us to pivot as an organization and ask where we can use this technology to do things others can’t and to really be quite transformative,” says Haurwitz. “Today, we’re a far more traditional drug development organization, with our initial focus on oncology and off-the-shelf cell therapies.”
Totally radical
Haurwitz strikes me as modest, yet she’s radical—her science is progressive and daring. After all, when Haurwitz took on CRISPR as a graduate student project in Jennifer Doudna’s lab at the University of California, Berkeley (UCB), the field was essentially nonexistent.
“This is what I call the dark ages of CRISPR,” Haurwitz recalled. “When I joined Jennifer Doudna’s lab, there were maybe three manuscripts that I could pull up on PubMed that were about CRISPR that postulated it was some sort of acquired immune system. Of those three, only one was an experimental paper, and the other two were bioinformatic. So, it was a fascinating time to get in on the ground floor and to contribute to some of the really basic understanding of the biology of this acquired immune system.”
Haurwitz didn’t have any information on how the CRISPR proteins work or how they come together. What she did know is that she didn’t want to be a professor, which went against the norm for most Doudna lab alumni.
“[Jennifer], Martin Jínek (postdoc), James Berger [UCB faculty at the time], and I got together and had a lot of conversations about what could be done that eventually morphed into what we could do,” says Haurwitz. “I had the incredible opportunity to jump in as the first operator and the founding CEO [of a CRISPR company].”
In 2011—one year before the landmark report co-authored by Doudna, Jínek, and members of Emmanuelle Charpentier’s lab that led to the 2020 Nobel Prize in Chemistry—Haurwitz founded Caribou. The name is a geeky, tongue-in-cheek reference, she admits—if you mash together “cas” and “ribo,” you can squeeze the word “caribou” out of it. The theme extends to the company’s logo—a caribou with RNAs for antlers.
The reckoning
Haurwitz must have had a career in finance in another life because, despite no other business experience on her resume, she runs a tight, de-risked operation at Caribou, and her business acumen is helping Caribou wade through some choppy waters. She only invests in a program if she believes it has best-in-class potential. “Nothing should be acute proof-of-concept; that’s a waste of everybody’s time and energy,” she says prudently.
After examining the cell therapy landscape, Haurwitz decided to focus Caribou on developing next-generation allogeneic CAR-T and CAR-NK cell therapies with significant potential to address the limitations of patient-derived treatments. “The autologous CAR T’s are extraordinary, and what they have done for patients is quite remarkable,” says Haurwitz. “Yet we just don’t see how that scales to truly deliver cell therapy for these broader patient populations. Off-the-shelf [therapies] has to be the answer.”
And not just off-the-shelf, healthy CAR-T and CAR-NK cells, but cells that are genetically manipulated to avoid immune rejection. They have to be armored, essentially, which Caribou is doing in various ways depending on the biology of the disease.
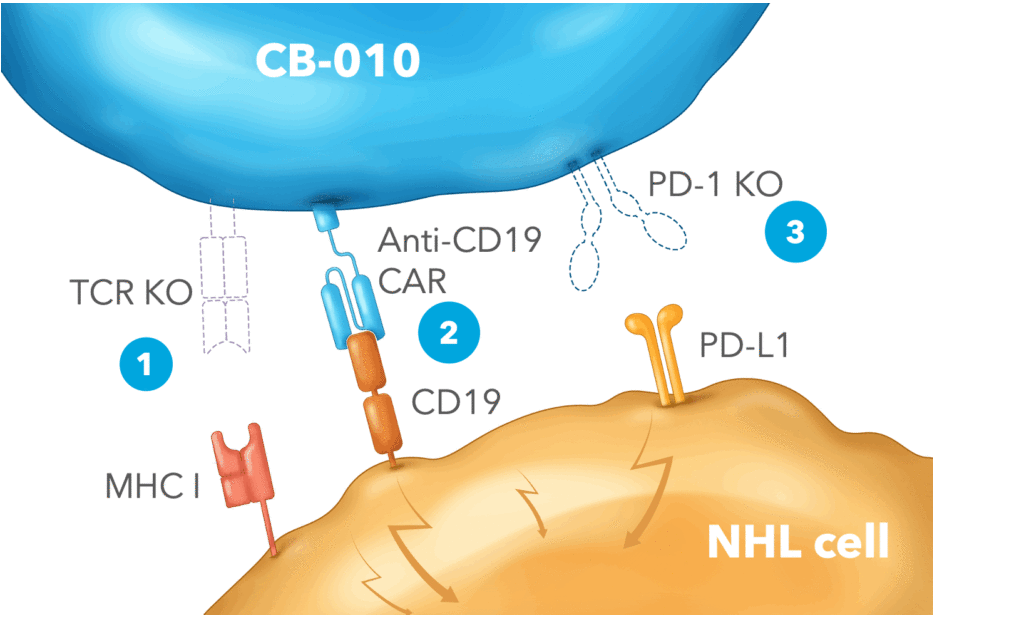
For example, in non-Hodgkin lymphoma, Caribou’s approach is a first-line, off-the-shelf cancer immunotherapy to get rid of the PD-1 checkpoint through genome editing called CB-010, which has recapitulated much of what has been seen in animal models, namely that the PD-1 knockout leads to a better therapeutic index.
As GEN reported in July 2021, Haurwitz took the company public, raising around $304 million. Her plan has been to advance the clinical development CB-010. To the best of Haurwitz’s knowledge, Caribou is the first company to put an allogenic CAR-T in the clinic with a PD-1 knockout and has publicly disclosed data on the first cohort of patients dosed. These six patients had a complete response with what is considered a low dose of CAR-Ts (40 million cells). “Even in a head-to-head comparison against the autologous CAR-Ts, no one has seen that kind of activity at such a low dose,” she says. “I think we’re onto something.”
In addition to CB-010, Caribou has initiated clinical and preclinical studies for its CAR-T cell therapies for relapsed/refractory multiple myeloma (CB-011) and relapsed or refractory acute myeloid leukemia (CB-012), respectively. Additionally, Caribou is continuing research and development of its iPSC-derived CAR-NK cell therapies for solid tumors, to continue the advancement of its genome-editing technologies, and for discovery-stage research of potential additional programs.
But that’s not enough. With her eyes on the evolving commercial landscape, Haurwitz is trying to predict where these cell therapies are going. While Caribou’s initial data on first-line off-the-shelf cancer immunotherapies are promising, Haurwitz wants to keep pushing the bounds of this technology.
Behind her conviction that autologous CAR-Ts will become the standard of care, Haurwitz has Caribou testing the efficacy of their allogenic CAR-Ts as a second-line treatment (the follow-up treatment for a disease after the initial first-line treatment has failed). “We have some very compelling initial data and a path into the second line that I think keeps us quite competitive in this landscape,” Haurwitz says.
Something tells me that Haurwitz has already thought a few steps ahead of this, with something already up her sleeve. But Haurwitz is not using sleight of hand; she’s playing chess, not checkers.
Human after all
As we wind down our conversation, I ask if she has any safety concerns about the use CRISPR, for it is after all an evolutionarily distant immune response and not an endogenous human mechanism.
Unfazed, she responds pragmatically: “You have to start by asking, ‘What disease are you trying to treat, what are the tools you bring to bear on it, and then what are the safety implications of the utilization of those tools?’ The work that we’re doing today is, as I’ve described, exclusively ex vivo, so we’re not delivering high doses of genome editing reagents into cells.”
Then, Haurwitz switches hats from practical CEO to curiosity-driven scientist. “It’s fun to step back and think about future uses of these technologies,” she said, anticipating the annual CRISPR conference coming up in Germany in a few weeks, which she’s only ever missed once (because she was “too pregnant to fly”).
As Haurwitz departs, I am struck by Sun Tzu’s famous quote from The Art of War: as swift as wind, as gentle as forest, as fierce as fire, as unshakable as mountain.
After all, Haurwitz is a force of nature.