Amgen Inc.
ll stakeholders involved in executing clinical trials during the pandemic faced enormous challenges, creating disruptions in clinical trial execution and delays in providing products to patients. The advice that global regulators provided to help address those issues was invaluable, and industry members are extremely grateful for that assistance. However, many different approaches were deployed by regulators across the globe. Those differences presented issues as industry tried to navigate the various requirements to continue clinical trials as safely as possible for patients. Many regulators are currently evaluating the impact of these flexible approaches and may continue to allow certain flexibilities in the future. This article highlights differences in regulatory guidance, suggests a deeper evaluation of the reasons for the various approaches, and advocates for appropriate alignment of guidances consistent with learnings from the pandemic.
One positive outcome of the clinical trial disruptions that resulted from the pandemic is that we found new ways to reach patients, incorporating their needs and preferences. We all now recognize and appreciate the positive impact of patient-centered approaches to trials and want to foster them. Greater harmonization will support the use and adoption of innovative clinical trial designs and clinical trial approaches. These include decentralized trials (use of digital and other technologies to reach patients in a non-traditional trial model), or a hybrid of traditional and decentralized trials. Those types of designs and programs are promising for the future of drug development. In particular, they can promote inclusive clinical trial participation, helping to reach a more diverse population.
Appropriate remote operations became critically important, including remote monitoring and digital evidence collection. The pandemic required industry to implement advanced tools and analytics more broadly and demonstrated a clear need for improved clinical trials post-pandemic. However, new regulatory guidances must be aligned more closely to successfully advance innovative and patient-centered clinical trials. We also need to harness the creativity and energy that emerged during the pandemic over the past year and a half, and build a better, more modernized clinical trial system to serve our patients.
Examples of Varying Approaches
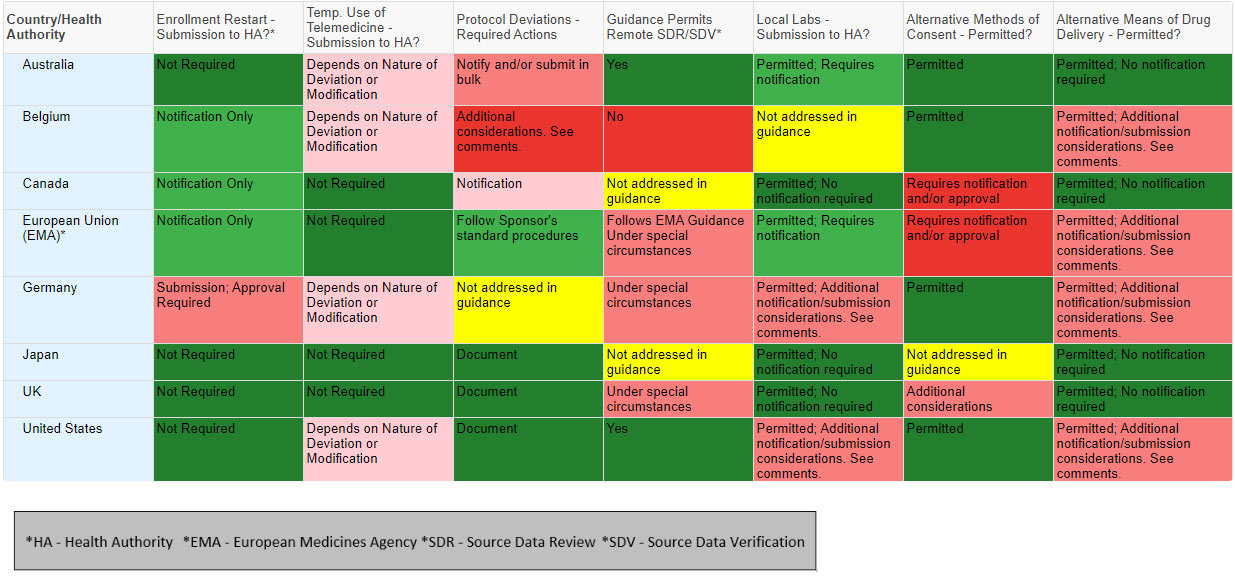
The chart is not intended as legal or regulatory advice; rather, they are a visual aid to understand the challenges that clinical research teams may encounter in different countries or regions. The visual aid proved helpful for internal discussions, and we believe that it can facilitate external discussions as well. Ideally, regulators will engage in a dialogue regarding the rationale behind the varying regulatory approaches and identify where those differences can be minimized or eliminated in the future. The chart below highlights a few key topics, from eight countries, to illustrate the issue.
The chart above represents a sample of variations by country related to a few key guidance topic areas. We developed similar charts for over 50 health authorities that can help highlight the issues and support a dialogue.
We understand that regulators are working to align approaches where possible and to learn from regulatory flexibilities and agilities during the COVID-19 crisis. That includes the use and regulatory acceptance of digital tools, decentralized approaches, telemedicine, and modern statistical methods, among others. Consistent with the good clinical practices mandate, regulators will need to review data from clinical trials that were affected by the pandemic, to determine which flexible regulatory approaches are appropriate to maintain. Industry needs to provide information for regulators to make those evidence-driven decisions and help inform the future of clinical trials. All stakeholders express the desire to move forward in aligning regulatory approaches. To be successful, approaches must be global. Dialogue and understanding of the impact of varying approaches will go a long way toward achieving the goal of improved, innovative, patient-centered clinical trials for the future.