Radical new skin cancer treatment shows promise in first clinical trials
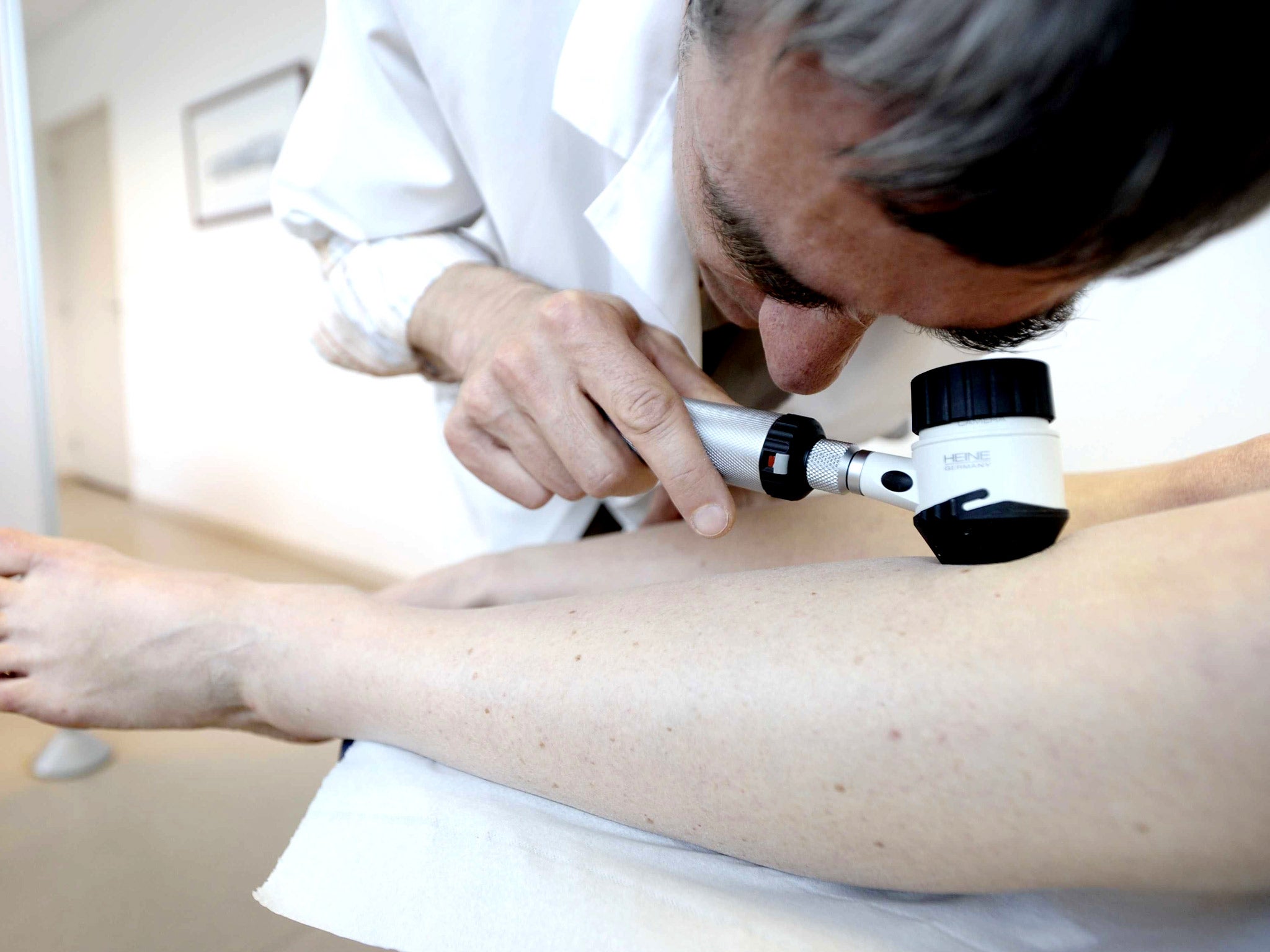
A radical new form of cancer treatment that relies on the body’s natural “killer cells” to attack tumours has proved a success in the first clinical trials on patients suffering from advanced skin cancer, scientists have said.
The immune-therapy is based on a biologically designed drug that binds tumour cells to the killer T-cells of the immune system, causing the cancer cells to self-destruct. Scientists hope that the approach can be adapted for a wide range of other tumours, such as prostate, lung and ovarian cancers.
Results of the phase 1 clinical trial designed to test the drug’s safety on 31 patients with advanced melanoma – the most dangerous form of skin cancer – showed there were few serious, long-lasting side effects and that in some cases the tumours started to shrink.
The findings, released at the American Association for Cancer Research in San Diego, California, were better than expected and have already led to a phase-2 clinical trial to test the drug’s efficacy on skin cancer patients in both the UK and the United States, scientists said.
“I think this is at the high end [of expectations]. A number of phase-1 trials go nowhere but what we see here is a drug that works as predicted and has significant clinical activity. It is very, very exciting,” said Mark Middleton, professor of experimental cancer medicine at Oxford University, who presented the results.
Of the 16 patients in the group who received clinically useful doses of the drug, four responded to treatment and two of these met the strict clinical criteria that are used to define whether or not a patient with solid tumours responds to an experimental treatment.
Two of the patients in the advanced stages of the disease have continued with the immune-therapy treatment on compassionate grounds and still show signs of benefiting from the drug, known as IMCgp100, nearly a year after the trial began.
“The drug is well tolerated in advanced melanoma patients, and we have seen clinical responses in some of them,” Professor Middleton said. Many of the patients experienced tumour inflammation and rashes, which indicates that their immune systems had been stimulated to start attacking the cancer cells within the skin, he said.
“The one aspect that did surprise us is the extent of tumour inflammation that is possible to achieve from just a single dose of the drug, because we thought it might take several weeks to get going,” Professor Middleton said.
“Although we are encouraged by the clinical activity observed, all of the available scientific and clinical data suggest that it should be possible to increase the level of clinical activity with an improved dosing regimen,” he said.
IMCgp100 is a small, man-made antibody designed specifically to bind tightly to the unique protein antigens sticking out from the surface of the cancer cell. At the other end of the synthetic antibody is another binding site that attaches to passing killer T-cells in the blood, bringing them close enough to destroy the cancer cells while hopefully leaving ordinary healthy cells unharmed.
The intellectual property behind the technology is owned by a small spinoff company based near Oxford University called Immunocore, which has over the past year or so signed major deals potentially worth hundreds of millions of pounds in future investment with three multinational pharmaceuticals companies.
Bent Jakobsen, the Danish-born chief scientific officer of Immunocore, who began working on the technology more than 20 years ago, said that the drug was well tolerated in the melanoma patients and any temporary side-effects, such as skin rash and fever, were anticipated and short-lived.
However, what was a surprise was just how well some of the patients responded, even though the trial was not designed with this in mind, with one individual showing an 80 per cent reduction in tumour size, Dr Jakobsen said.
“Some tumour shrinkage was over 30 per cent, others were less than 30 per cent, but the drug was not designed to work after a single dose so in some respects this was a surprise to us,” Dr Jakoben said.
“Right now I find it very, very encouraging to get these results. Some patients have responded well and continue to respond,” he said.
The next stage is a phase 2a trial to be carried out on other patients with melanoma to test the best dose regimes – weekly or daily – and, following this, a final, phase-3 trial may be set up to assess whether the drug could be offered alongside or instead of existing treatments.
Immune-therapy to combat cancer is widely seen as one of the most exciting and novel approaches to fighting the disease because it uses the body’s own natural defences designed to battle bacterial and viral invaders. The problem with treating cancer is that it’s a disease caused when the machinery of the body’s own cells goes awry.
Subscribe to Independent Premium to bookmark this article
Want to bookmark your favourite articles and stories to read or reference later? Start your Independent Premium subscription today.

Join our commenting forum
Join thought-provoking conversations, follow other Independent readers and see their replies