First principles approach to creating new materials
Traditionally, scientists discover new materials, and then probe them to try to better understand their properties. Theoretical materials physicist Craig Fennie does it in reverse.
"We have been rethinking the problem of materials discovery from that of the perspective of a physicist," says Fennie, a National Science Foundation (NSF)-funded scientist and a 2013 recipient of a prestigious MacArthur fellowship, a $625,000 no strings attached award, popularly known as a "genius" grant.
Scientists typically "make something, measure it, report it, then try to understand what they just reported. Today we are turning that approach around," he says, essentially by combining the tools of theoretical physics with those of solid-state chemistry to discover new materials with attractive and useful electrical, magnetic and optical properties. "Pioneers in my field...taught us that we (physicists) can and should do more."
Fennie, an assistant professor of applied and engineering physics at Cornell University, is creating new materials by employing a "first principles" approach based on quantum mechanics, in which he builds materials atom by atom, starting with mathematical models, in order to gain the needed physical properties.
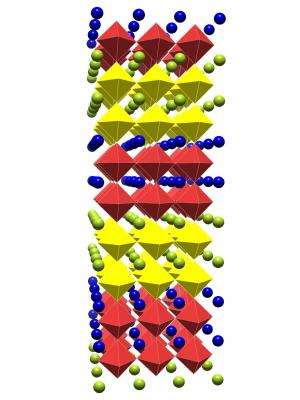
A main focus of his research is in understanding how the composition, geometry and topology of complex crystalline motifs influence the interplay among lattice, magnetic, orbital and/or electronic degrees-of-freedom, how this subsequently manifests itself in the macroscopic properties, and if controlling this interplay can produce designer properties and functionalities.
One application of this strategy has been to develop materials with coupled electrical and magnetic states, which opens the possibility of controlling digital data storage electronically rather than magnetically.
His theories have indicated, for example, that a europium titanium oxide, when stretched, would exhibit unusually strong coupled electric and magnetic properties; the resulting material could lead to dramatic advances in memory storage capacity, that is "anything where you would need memory," he says.
Fennie combines microscopic models, known as Hamiltonians, with fundamental principles of solid-state chemistry and symmetry to develop a set of design criteria to aid in identifying a real material with the physics of interest built-in, a process that is "the creative and imaginative aspect of our work," he says. He then tests the materials viability on the computer.
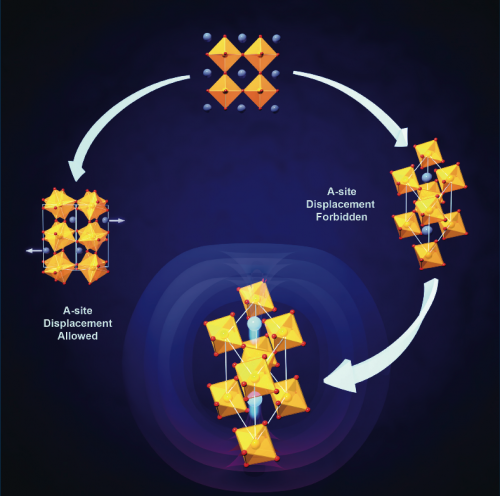
"How we arrive at an initial new material is often driven by intuition, but first principles techniques, such as density-functional theory, allow us to screen materials to test whether or not they in fact display the design criteria," he says.
Only then does he turn to expert scientists to actually make the materials. "Job half done when the theory paper is written," he says, adding: "I'm not satisfied until we know this material can be synthesized and has the properties we think they should have."
For this, he usually turns to Cornell colleagues, among them Darrell Schlom, professor of industrial chemistry in the materials science department, who is the "grower," and David Muller, professor of applied and engineering physics, who is Schlom's "eyes," Fennie says.
"Darrell makes just about everything I have worked on, while David, using his powerful electron microscopes, is able to see where Darrell is putting the atoms," Fennie says. "I could not do what I do without them.
"As physicists, we are really interested in discovering new phenomena," he adds. "But just because the phenomena, or property, already might be known, we believe the fundamental aspect of the problem is not over. It's a lot like the purest theoretician's saying, 'we can calculate, therefore we understand.' For us, we would argue that it isn't until we can design it and see it realized in real life that we truly understand something. So the issue is: how do you build up a real material around a model, or property?"
Fennie is conducting his research under an NSF Faculty Early Career Development (CAREER) award, which he received in 2011. The award supports junior faculty who exemplify the role of teacher-scholars through outstanding research, excellent education, and the integration of education and research within the context of the mission of their organization. NSF is funding his work with $400,000 over five years.
He also receives, over four years, approximately $280,000 in additional NSF funding through the Designing Materials to Revolutionize and Engineer our Future (DMREF) program. In this program, centered at the University of Wisconsin- Madison and led by Chang-Beom Eom, a professor of materials science and engineering, Fennie is focusing on identifying new atomic-scale interface materials.
At Cornell, Fennie also is part of the Cornell Center for Materials Research, an NSF funded Materials Research Science and Engineering Center (MRSEC). "I've used the opportunity provided by NSF's MRSEC program to move, albeit more slowly than I would have liked, in a few new directions, namely in that of correlated metals and in spintronics," he says, referring to an emerging technology that uses the spin of the electron, its magnetics and electronic charge in solid state devices.
As part of the CAREER grant's educational component, Fennie's goal is to recruit and retain more women and underrepresented minority students in physics by having them become more involved in physics research. Given his own convoluted route, Fennie hopes to show by example "that scientists aren't born as such, and that it's never too late," he says.
"On paper, I should never have gotten as far as I have," he adds, a reference to the rough Philadelphia neighborhood where he was born and grew up. "But my parents, who did not go to college, showed me how to work hard and taught me never to accept others perceived limitations of oneself."
He served for three years as the Cornell Center for Materials Research faculty advisor to NSF's Research Experience for Undergraduates (REU) program, which provides opportunities for undergraduates from other schools to participate in hands-on interdisciplinary materials research projects involving chemistry, physics, materials science, and engineering disciplines, where such opportunities are usually not available at their home colleges.
He is, in fact, an REU alum himself. "I really want other students like me to see what research in the sciences is like," he says. "I studied electrical engineering as an undergrad and although the place I studied at is an excellent place to study engineering, there was not a lot of science research going on.
"I did a REU at Cornell in the materials science department and it opened my eyes to what interdisciplinary research in the sciences is all about," he adds. "I've been hooked ever since."
Provided by National Science Foundation