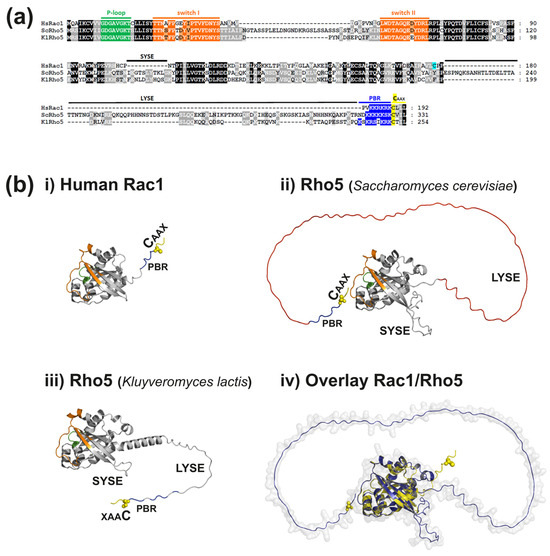
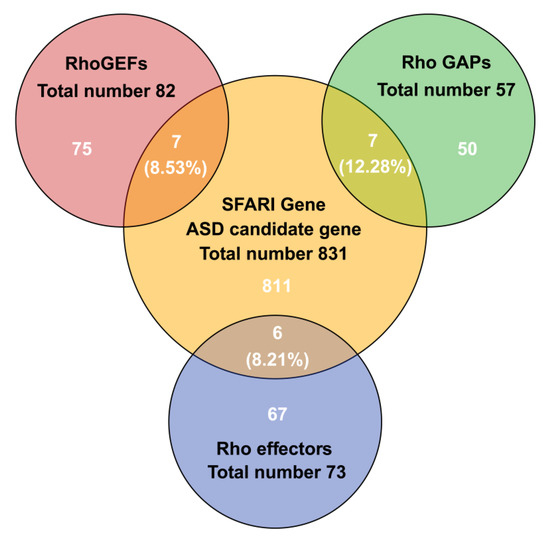
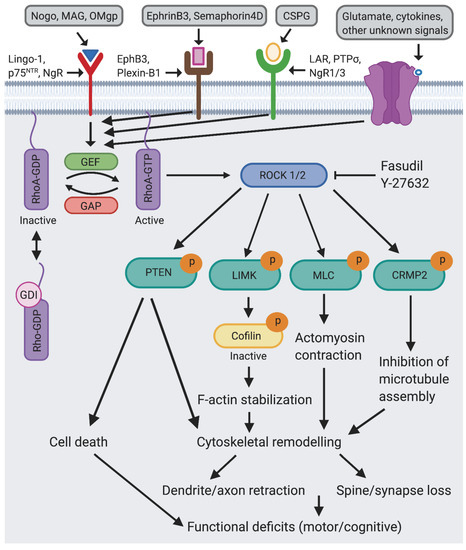
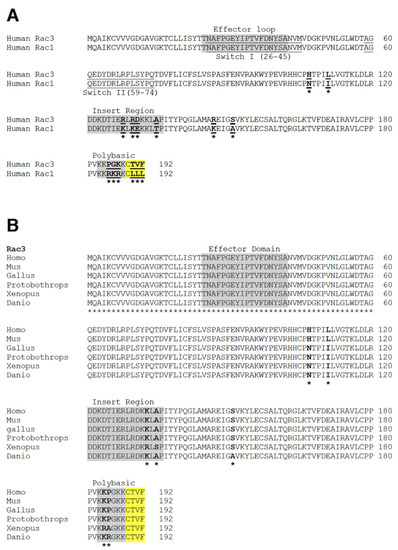
Rho GTPases in Health and Disease
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Signaling".
Viewed by 128781Editor
Interests: Rho GTPases; keratinocytes; mouse disease models
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
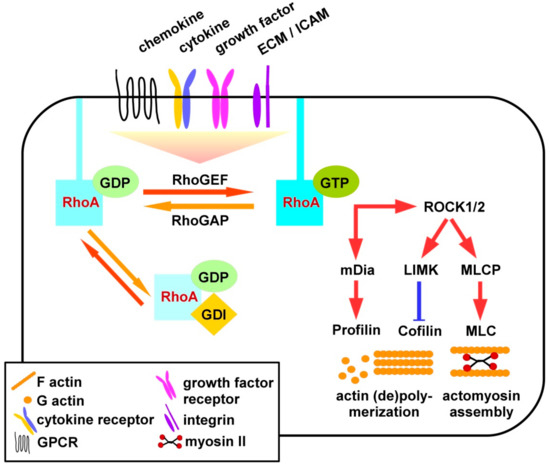
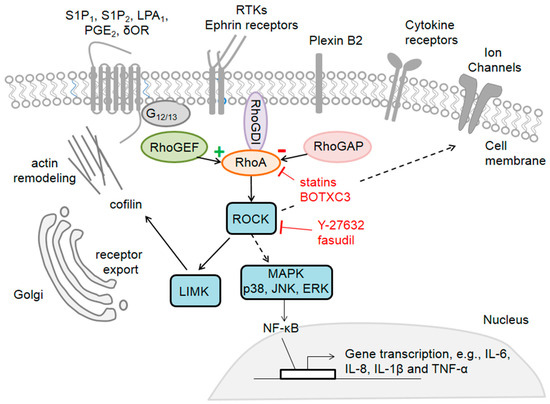
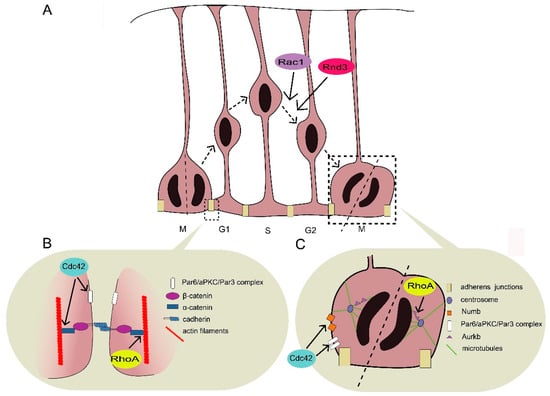
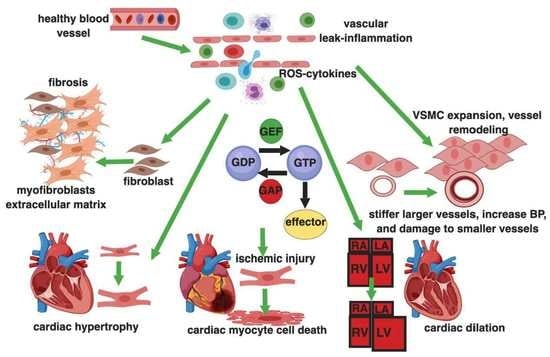
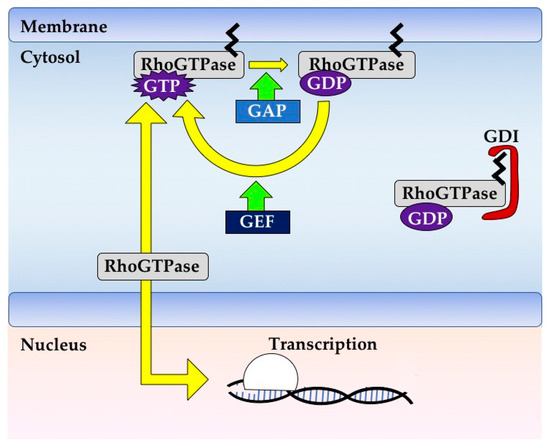
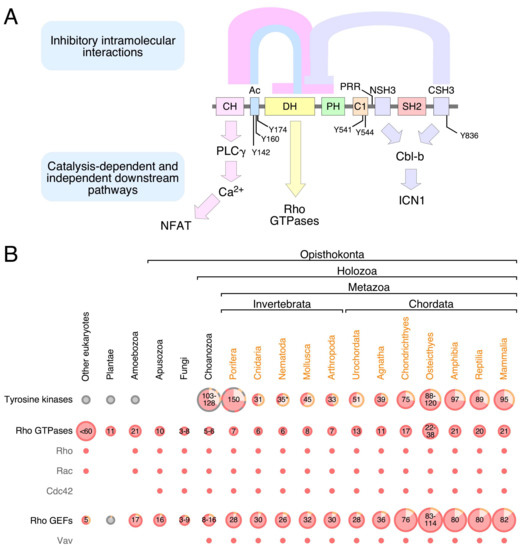
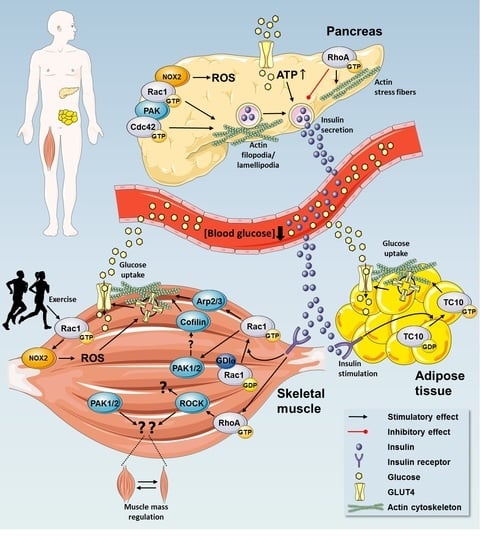
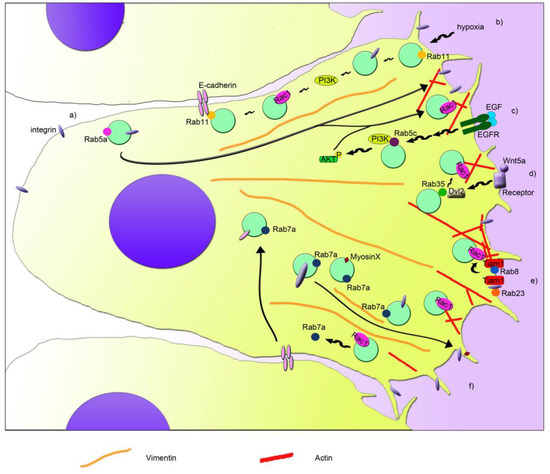
Rho GTPases are crucial organizers of the actin cytoskeleton with essential functions in cell migration and cell–cell contacts. In addition, Rho GTPases are involved in the regulation of stemness, proliferation and differentiation, and other cellular processes. Not surprisingly, this exciting family of molecules plays important roles in development and in disease. Genetically modified mice provided, in the last few years, important and often surprising insights into the in vivo function of Rho GTPases, and cellular studies in vitro suggested novel molecular mechanisms underlying the observed effects.
Still, many questions remain to be investigated in order to understand Rho GTPase function in development and disease. For example, what is the role of Rho GTPase crosstalk in vivo? What is the importance of posttranslational modifications of Rho GTPases? How crucial is the parallel activation of different effector pathways by an activated Rho GTPase? How is Rho GTPase function in 3D systems related to 2D models, the currently preferred system for the molecular analysis of Rho GTPase signalling?
For this Topical Collection of Cells on “Rho GTPases in Health and Disease” we invite scientists now to contribute review articles and primary research papers on the subject. Ideally, the review articles should, not only describe the current state-of-the-art, but also give personal opinions about future challenges and research goals in that area.
We hope that such a Topical Collection will make the scientific community aware of possible links of their work to Rho GTPases, which will trigger fruitful discussions, and that it will motivate young researchers to enter the Rho GTPase field and tackle the open questions of Rho GTPase functions in health and disease.
Dr. Cord Brakebusch
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Rho GTPases
- Actin cytoskeleton
- In vivo disease models
- Rho GTPase regulation
- 3D in vitro models
- Development