The Overlooked Organisms That Keep Challenging Our Assumptions About Life
Gorgeous and weird, lichens have pushed the boundaries of our understanding of nature—and our way of studying it.
Science is sometimes caricatured as a wholly objective pursuit that allows us to understand the world through the lens of neutral empiricism. But the conclusions that scientists draw from their data, and the very questions they choose to ask, depend on their assumptions about the world, the culture in which they work, and the vocabulary they use. The scientist Toby Spribille once said to me, “We can only ask questions that we have imagination for.” And he should know, because no group of organisms better exemplifies this principle than the one Spribille is obsessed with: lichens.
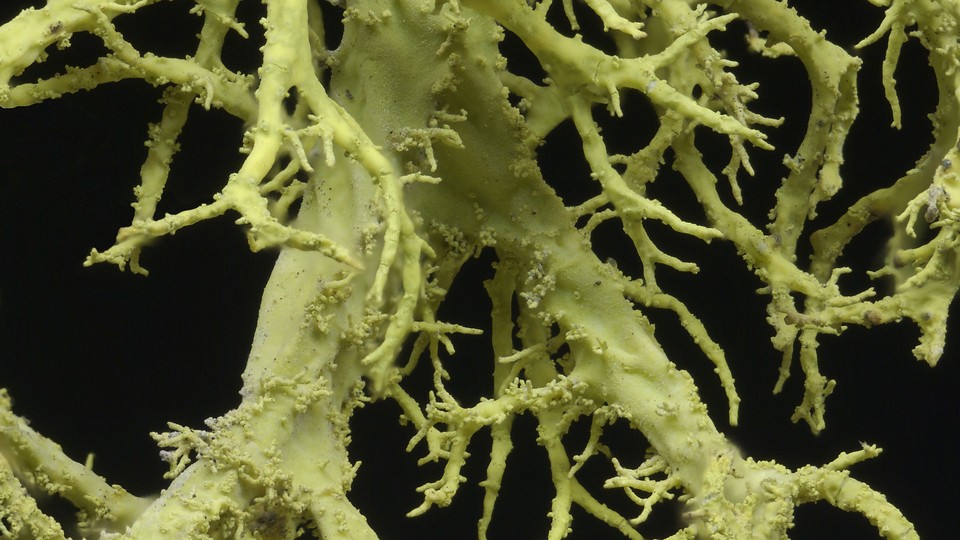
Lichens can be found growing on bark, rocks, or walls; in woodlands, deserts, or tundra; as coralline branches, tiny cups, or leaflike fronds. They look like plants or fungi, and for the longest time, biologists thought that they were. But 150 years ago, a Swiss botanist named Simon Schwendener suggested the radical hypothesis that lichens are composite organisms—fungi, living together with microscopic algae.
It was the right hypothesis at the wrong time. The very notion of different organisms living so closely with—or within—each other was unheard of. That they should coexist to their mutual benefit was more ludicrous still. This was a mere decade after Charles Darwin had published his masterpiece, On the Origin of Species, and many biologists were gripped by the idea of nature as a gladiatorial arena, shaped by conflict. Against this zeitgeist, the concept of cohabiting, cooperative organisms found little purchase. Lichenologists spent decades rejecting and ridiculing Schwendener’s “dual hypothesis.” And he himself wrongly argued that the fungus enslaved or imprisoned the alga, robbing it of nutrients. As others later showed, that’s not the case: Both partners provide nutrients to each other.
Today, such a relationship is called a “symbiosis,” and it’s considered the norm rather than the exception. Corals rely on the beneficial algae in their tissues. Humans are influenced by the trillions of microbes in our guts. Plants grow thanks to the fungi on their roots. We all live in symbiosis, but few organisms do so to the same extreme degree as lichens. If humans were to spend their lives in the total absence of microbes, they’d have many health problems but would unquestionably still be people. But without its alga, a lichen-forming fungus bears no likeness to a lichen. It’s an entirely different entity. The lichen is an organism created by symbiosis. It forms only when its two partners meet.
Or does it?
Lichen-forming fungi mostly belong to a group called the ascomycetes. But in 2016, Spribille and his colleague Veera Tuovinen, of Uppsala University, found that the largest and most species-rich group of lichens harbored a second fungus, from a very different group called Cyphobasidium. (For simplicity, I’ll call the two fungi ascos and cyphos). The whole organism resembles a burrito, with asco fillings wrapped by a shell that’s rich in algae and cyphos.
For many, it was a game-changing discovery. “The findings overthrow the two-organism paradigm,” Sarah Watkinson of the University of Oxford told me at the time. “Textbook definitions of lichens may have to be revised.” But some lichenologists objected to that framing, arguing that they’d known since the late 1800s that other fungi were present within lichens. That’s true, Spribille countered, but those fungi had been described in terms that portrayed them as secondary to the main asco-alga symbiosis. To him, it seemed more that the lichens he studied have three core partners.
But that might not be the whole story, either.
Look on the bark of conifers in the Pacific Northwest, and you will quickly spot wolf lichens—tennis-ball green and highly branched, like some discarded alien nervous system. When Tuovinen looked at these under a microscope, she found a group of fungal cells that were neither ascos nor cyphos. The lichens’ DNA told a similar story: There were fungal genes that didn’t belong to either of the two expected groups. Wolf lichens, it turns out, contain yet another fungus, known as Tremella.
This isn’t entirely new. Over the years, other lichenologists have detected Tremella in wolf lichens, but only ever in three specimens, and only in the context of abnormal swollen structures called galls. “It was thought to be a parasite,” Tuovinen says. “But we found it in completely normal wolf lichens that don’t have any kinds of bumps.” Tremella is right there in the shell of the lichen burrito, next to the cyphos. It seems to make extremely close contact with the algae, hinting at some kind of intimate relationship. And it’s everywhere. Tuovinen analyzed more than 300 specimens of wolf lichens from the U.S. and Europe, and found Tremella in almost all of them.
Wolf lichens are among the most intensively studied of all lichens, so how could such a ubiquitous component have been largely missed? The problem, Tuovinen says, is that under a normal microscope, “the fungal cells all look the same.” She saw it only when she tagged the lichens with glowing probes that were designed to recognize Tremella genes. And she knew to do that only after finding those genes amid wolf lichen DNA. Earlier genetic studies, she says, might have missed them because they had specifically focused on the genes of the ascos. “There hadn’t been a reason to expect anything else based on the knowledge at the moment,” she says.
It’s an exciting discovery, says Erin Tripp, a lichenologist from the University of Colorado Boulder, but it’s still unclear what Tremella is actually doing. Most likely, she argues, it’s an infection, albeit a very widespread one. The alternative is that Tremella is a core part of the lichen. “This would, of course, be very exciting,” Tripp says, but to demonstrate that, the team would need to try to reconstitute wolf lichens with or without Tremella or, alternatively, use gene-editing techniques to disable the fungus and check how the lichens respond. “Without this sort of experimental approach, it seems premature to suggest that Tremella represents a third, fourth, or whatever-th symbiont.”
Tuovinen agrees that one shouldn’t overplay Tremella’s role. But she argues that lichenologists have too readily downplayed such organisms. More than 1,800 species of non-asco fungi have been described within lichens, and they’ve been labeled with terms that imply some kind of externality: commensalistic. Parasymbiotic. Endolichenic. Lichenicolous. If they’re not ascos, “we somehow just decided, without testing, that they’re parts of a lichen that can be excluded,” Tuovinen says. “We really don’t know that.”
“Language matters a lot when dealing with these organisms,” Spribille, now at the University of Alberta, adds. “If we set up our language so that our definition of a lichen is fixed, and these other elements are extrinsic, we’re setting ourselves up to find that they’re extrinsic.” He thinks that researchers should move away from “the imperative of classification” and the compulsion to shoehorn organisms into fixed buckets. He suspects that the relationships between all the components of a lichen are probably highly contextual—beneficial in some settings, neutral or harmful in others.
That’s a lesson other scholars of symbiosis should also heed. There’s a tendency to categorize the bacteria within an animal’s microbiome as good or bad, as beneficial mutualists or harmful pathogens. But such labels imply an inherent nature that likely doesn’t exist. The same microbes can be benign or malign in different contexts, or perhaps even at the same time. Biology is messy—as are lichens.
Tripp agrees that “we, as a community of lichen biologists, need to revisit the role of all symbionts in the lichen microcosm.” No matter how one describes Tremella and other lichen-associated fungi, it’s clear that they do affect the form and function of the lichen as a whole. How they do so is “the great unsolved problem” of lichenology, says Anne Pringle of the University of Wisconsin at Madison. “Are the multiple species of fungi interacting mutualistically? With each other? With the algae? Are some parasites? Probably the answer to all questions is yes. Regardless, the data support an emerging consensus: Lichens are ecosystems as well as organisms.”
How many partners are there in a lichen? “I don’t know, but I think it depends on the lichen,” Spribille says. “I don’t expect there to be any one configuration that makes a lichen, a lichen.” That’s especially likely because lichens have evolved many times over, from different lineages of ascos that independently formed partnerships with different algae, over hundreds of millions of years. To expect them all to share the same basic plan is like expecting birds to be the same as fish.
They’re especially hard for us to understand because they’re so different from the organisms we’re familiar with. Unlike animals and plants, lichens don’t really have tissues. They don’t grow from embryos, and instead form through fusion. Different combinations create different forms—brittle or flexible, flat or round—and these traits are likely just as important to them as wings or legs or eyes are to animals. “We don’t understand their needs,” Spribille says. “In the absence of that, it’s difficult to say what kinds of configurations are within the realm of the possible.” And we can only ask questions that we have imagination for.