Scientists end long-standing controversy about a ubiquitous reaction involved in catalysts, corrosion, and more
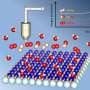
ater is behind creating certain biofuels, sequestering carbon, and forming corrosive rust. If and how water (H2O) breaks when it hits a metal oxide surface, such as a catalyst or a pipe, matters. In a pioneering study, scientists at Pacific Northwest National Laboratory (PNNL), led by Dr. Zdenek Dohnálek and Dr. Roger Rousseau, definitively measured the stability of adsorbed water compared to the hydroxyl (-OH) fragments. They showed that there is a slight preference to keep water molecules intact. The research team showed that when they do break, it is because surface forces align water molecules in a specific way, long before they hit the surface and dissociate.
For decades, scientists studied the reactions between water and oxides. They obtained substantial amounts of data; however, it did not definitively resolve water's stability, or how much energy is needed to make the water molecules fall apart. The PNNL team ended the controversy by precisely measuring stability of water and its fragments; they found that molecular water is only 0.035 eV more stable than its dissociated counterparts (hydroxyl groups). Supplying the definitive answer for the scientific community will benefit those working to efficiently and effectively improve the catalysts involved in producing fuels and chemicals.
"The approach lets us look at how bonds break in catalysis," said Dohnálek, who led the experimental research. "However, it applies much more broadly. Any dissociative process that has an energy barrier will benefit from this study."
Directly measuring the amount of energy needed to split bonds inside a water molecule when it hits an oxide surface was a major challenge. The team at PNNL spent several years getting the exact answer. "The problem wasn't that there wasn't enough data," said Rousseau. "Everyone had tons of answers. They just weren't what we needed."
To determine water's stability on titanium dioxide, a prototypical surface, experts in surface science and chemical theory came together. Because conventional instruments couldn't measure water's stability, the team first designed an instrument able to measure the energy and see the collisions. They combined a supersonic molecular beam with scanning tunneling microscopy and froze the molecular fragments by working at low substrate temperatures. It allowed the scientists to distinguish the molecules from the fragments.
By iterating between experiments and simulations, the team made numerous discoveries about the way water interacts with the oxide. For example, they found that the oxide surface influences the water molecules from relatively far away, which means a few molecular lengths in this case. While such "steering" of the molecules is seen on metal surfaces, it occurs at much shorter distances.
"When I saw the data, it hit me that this can only be explained by looking at how the water molecules approach the surface," said Dr. Vanda Glezakou, who suggested this analysis.
In the end, they determined that when the water fragmented to form two hydroxyl groups, the amount of energy needed to activate the bond that splits off the proton is relatively small, just 0.36 eV and the amount of energy to put the water back together is only 0.035 eV smaller, showing that water is only tiny bit more stable.
The team's technique can provide answers on other systems where bond activation is crucial, including in biomass production and carbon storage.
The PNNL team is applying the approach to catalytic problems as part of the Institute for Integrated Catalysis. Specifically, they are studying how molecules other than water break on surfaces. They are examining the different intermediate products formed. Further, they are seeing how heat influences dissociation in different reactions.
More information: Zhi-Tao Wang et al. Probing equilibrium of molecular and deprotonated water on TiO(110), Proceedings of the National Academy of Sciences (2017). DOI: 10.1073/pnas.1613756114
Journal information: Proceedings of the National Academy of Sciences
Provided by Pacific Northwest National Laboratory