Biochemists solve mystery of how DNA opens up to be copied
For years, scientists have puzzled over what prompts the double-helix of DNA to open its two strands and start replication. Knowing this could be the key to understanding how organisms — from healthy cells to cancerous tumors — replicate and multiply for their survival.
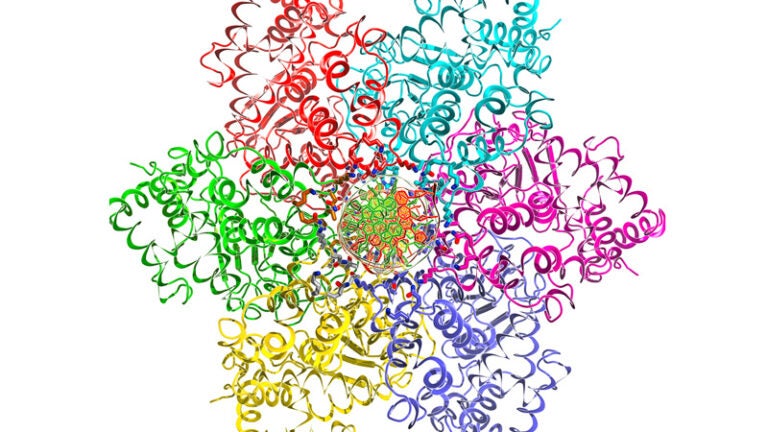
USC scientists believe they have solved the mystery. Replication is prompted by a ring of proteins that bond with the DNA at a special location known as “origin DNA.” The ring tightens around the strands and melts them to open up the DNA, initiating replication.
This all takes place at the nanometer level and is impossible to see with the naked eye. A strand of DNA is only about one nanometer in size — not even close to the width of a human hair, which is roughly equivalent to 100,000 DNA strands.
The researchers made their discovery by studying a virus called SV40. The found that the virus hijacked the DNA replication process with a ring of proteins called a helicase that mimicked the rings of proteins that prompt genetic replication in healthy cells.
“Understanding the mechanisms of origin DNA opening or melting allows us to learn this fundamental process of genetic duplication,” said corresponding author Xiaojiang Chen, professor of biological sciences and chemistry at USC Dornsife and director of the College’s Center of Excellence in NanoBiophysics. “The knowledge we have gained may be applicable for future intervention of this process to block the replication of viral pathogens and cancer cells.”

Xiaojiang Chen, director of USC Dornsife’s Center of Excellence in NanoBiophysics. Photo by Peter Zhaoyu Zhou.
Xerox copies
When the origin DNA melts, the double helix divides into separate strands, Chen explained. Those DNA strands then become the template for faithful duplication of other strands — a Xerox copy of their parental DNA. As soon as replication is complete, one double helix of DNA now becomes two exact copies of the same double helix.
“DNA replication is critical for heredity and survival,” said Chen, who also is affiliated with the USC Norris Comprehensive Cancer Center at Keck School of Medicine of USC. “The origin DNA’s opening is an essential step for DNA replication in our cells and for some tumor viral pathogens to replicate and spread.”
Why is origin DNA so special? Regular DNA sequences contain each of four components called nucleotides — designated A, T, G and C — in roughly equal measure. But origin DNA sequences contain more A and T nucleotides than usual.
To prompt replication, the scientists used a helicase from a “large tumor antigen,” or Large T. The antigen comes from the SV40 virus linked to human cancers such as brain and bone cancers, mesothelioma and lymphoma. The six proteins from Large T make up a helicase that mimics the structure of the healthy cells’ helicases.
The scientists obtained a 3-D view of the atomic structure of the helicase using X-ray crystallography, a technique for examining biomolecules and their structures at the atomic level. Chen said the images revealed that the proteins surrounding the DNA had attached to it, then tightened like a vice until the bonds between the two strands of the double helix broke — or melted — the origin DNA.
Although the scientists used a virus to study replication, healthy cells replicate in a similar way, Chen said.
Other co-authors on the study, which was published in the journal eLife, were USC Dornsife’s Dahai Gai and Shu-Xing Li and Damian Wang of Keck School of Medicine.
The study was funded by a National Institutes of Health grant (A1055926).