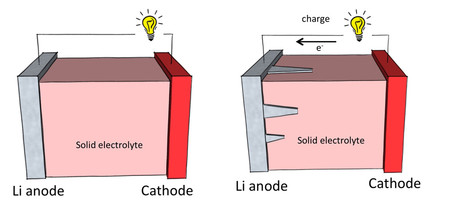
Lithium metal, which can store more energy than carbon, would seem to be an ideal anode material, except for one serious flaw: dendrites, tiny needle-like branching structures that can grow through the battery, eventually causing a short circuit.
Now, a joint team of researchers from Caltech and Carnegie Mellon University has measured the strength of lithium metal at the nano- and microscale, an achievement with important implications for suppressing dendrite formation.
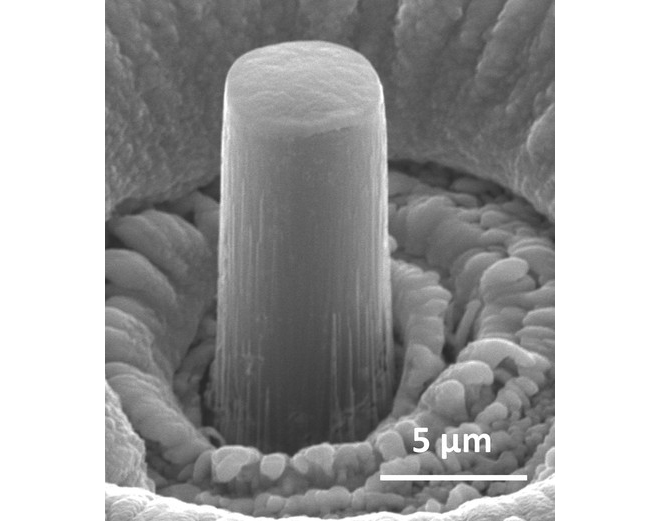
In “Enhanced strength and temperature dependence of mechanical properties of Li at small scales and its implications for Li metal anodes,” published online in the Proceedings of the National Academy of Sciences, Julia R. Greer and co-authors explain how they used a special vacuum chamber to form pillars of single-crystal lithium a few micrometers tall. The Caltech researchers discovered that at this size, lithium is up to 100 times stronger than previous measurements indicated. Collaborators at Carnegie Mellon University calculated how the stiffness of lithium dendrites varied with the crystallographic orientation and discovered that it could be as different as a factor of four.
“Lithium has historically been difficult to study because it oxidizes and immediately turns black upon contact with air,” Greer says. “Also, the strength of lithium has only been measured at a large scale, with some of the measurements dating back to the 1960s. Lithium dendrites are nano- to micrometer sized.”
Previous attempts to stop lithium dendrite growth have relied upon the use of a solid electrolyte, located between the cathode and anode, to physically suppress the dendrites.
“Physical suppression of dendrites with a solid electrolyte is a promising method, but thus far the electrolytes used have not been able to withstand the force of growing dendrites,” says co-author Chen Xu. “However, we now know that lithium dendrites are much stronger than previously thought, and we can choose a stronger solid electrolyte accordingly, which dendrites will be unable to grow through.”