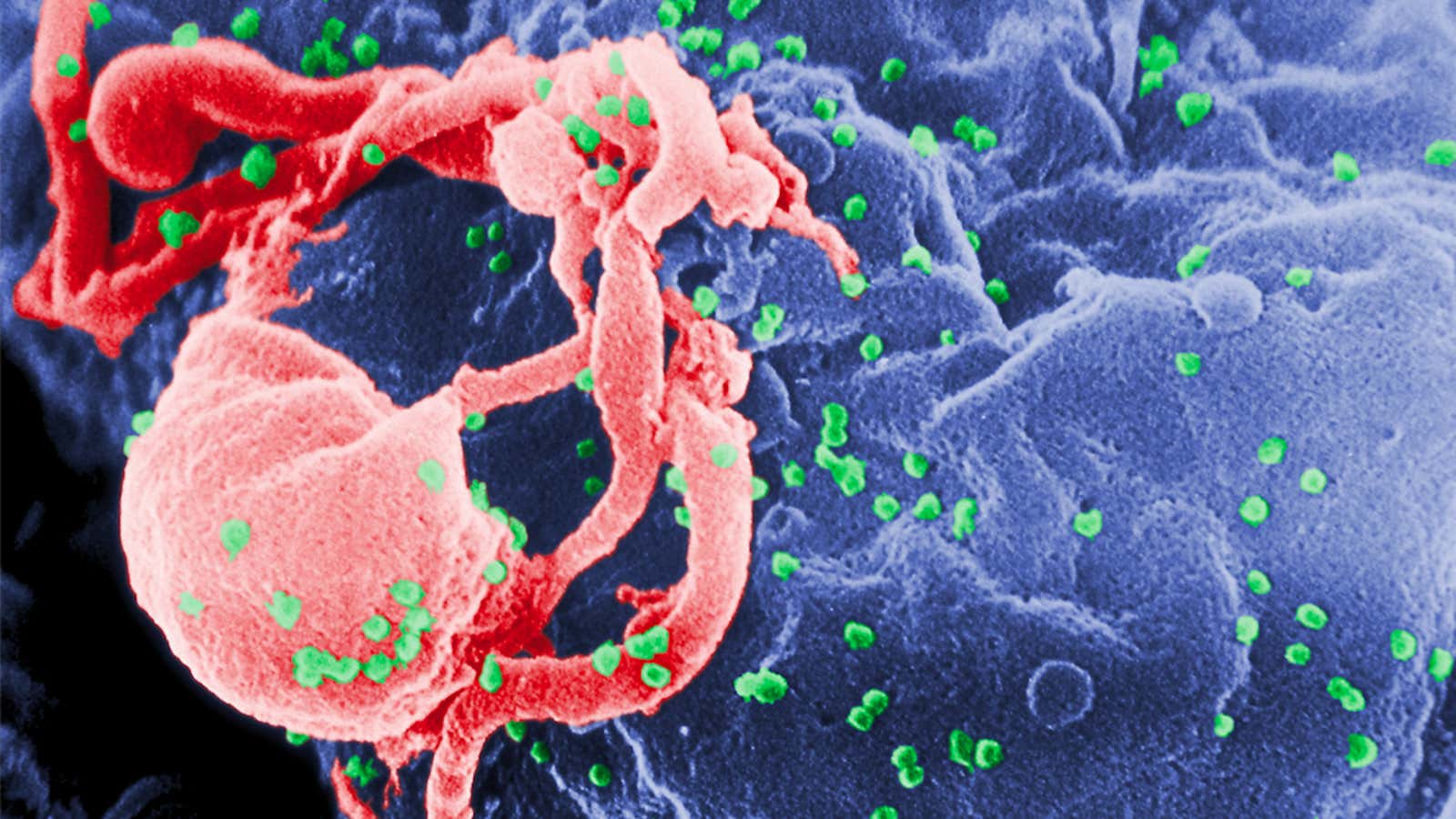
When one strain of the human immunodeficiency virus (HIV) made the leap from chimps to people in the early 1900s, it wasn’t the first time a virus of its kind had evolved to cross species borders. But it was the most significant in our history—to date, the pandemic has affected 70 million people worldwide, killing half of them.
HIV is a type of virus called a retrovirus, which are fairly common in all kinds of animals, including humans. Scientists had thought these viruses evolved quickly, and have been around for 100 million years or so. However, new research from Oxford University suggests that actually, these viruses have been around for closer to half a billion years—dating back to around the same time the first vertebrates were making their way from water onto land.
The fact that these viruses are so much older than previously believed means they’ve been adapting to evolutionary immune systems for much longer than we thought. “The emergence of retroviruses more or less coincides with the evolution of the vertebrates’ immune system and many of the other innovations of vertebrate immunity,” says Aris Katzourakis, a paleovirologist at Oxford University in England and lead author of the paper, published Jan. 10 in the journal Nature Communications.
In other words, these viruses have been co-evolving with animal (and, eventually human) immune systems since about as long as animals have been around: when viruses figure out how to make a host sick, the host immune system figures out how to thwart them until the viruses adapt again to the new changes. This back and forth has been has been something of an “‘arms race’ spanning hundreds of millions of years,” says Katzourakis.
Retroviruses leave a clear trail of their evolutionary history: They borrow a host cell’s enzymes to copy their single strand of genetic material and hide it within the host cell’s DNA. When the cell does get around to copying its own code, it mistakenly copies the virus’ imposter genes, too. Eventually, the viruses burst from the cell, sometimes killing it, but not always. Every now and then, viruses will insert their genetic code into sex cell DNA, and, in these cases where the extra code isn’t fatal, it can be passed down through generations of animals. Some of our own immune system, it turns out, has been shaped by genes from retroviruses.
Katzourakis and his team went digging through ancient genomes of fish and amphibians to find evidence of a type of retroviruses called “complex foamy viruses” (complex because of their larger genomes, like HIV; foamy because when they burst through the cell, they literally make it foam). They weren’t looking through actual fossils, but rather through massive files online. “It’s kind of like an online treasure hunt,” he says. “People sequence animal genomes all the time, and deposit them in open-access repositories on the internet.”
They looked within these internet genome archives for pieces of genetic code they knew came from different types of foamy viruses using a unique model that accounted for their known evolutionary rate. The new technique led to the discovery of evidence of ancient foamy viruses in fish and amphibian species dating back to between 460 and 550 million years ago.
Katzourakis thinks the best way for humans to get ahead in the animal-virus arms race is to study how these viruses have infected and become integrated into different hosts over the course of many years—that, he says, should lead scientists to discovering more examples of tricks immune systems have developed to beat them in the past. Mice, for example, have some genes (paywall) that block retroviral DNA from being inserted into their own. By looking for more of these sorts of immunity boosters, perhaps scientists can derive new ways to fight viruses like HIV.