No sperm or egg required: mouse proto-embryo made in the lab

Scientists have for the first time created embryo-like structures in the lab from stem cells, without recourse to eggs or sperm, they reported Wednesday.
In experiments, bundles of mouse stems cells—one type corresponding to the placenta, another to the embryo—self-organised into proto-embryos and initiated pregnancies when implanted into mouse wombs.
The procedure was not expected to create a viable embryo and did not do so, but could yield important insights into fertility and the earliest phases of life, according to a study published in the journal Nature.
"This breakthrough has opened up the black box of early pregnancy," said lead author Nicolas Rivron, a researcher at MERLN and Hubrecht Institutes in Utrecht, The Netherlands.
At the initial stage of development an embryo is about the width of a human hair and tucked inside the womb, making it inaccessible for in vivo research, at least in humans.
"These early embryos have all the cell types required to form a whole organism," said Rivron.
"They will help us better understand the hidden processes at the start of life, to find solutions for fertility problems, and to develop new drugs without the use of lab animals."
Currently, some two-thirds of in vitro fertilisation (IVF) treatments fail, mostly at the time of implantation in the uterus. Why remains largely unknown.
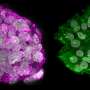
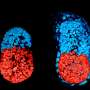
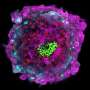
A few days after a mammal egg has been fertilised, it develops into a so-called blastocyst, a hollow sphere formed by less than 100 cells divided into an outer layer—the future placenta—and a small cluster in the middle, the future embryo.
Individual stem cell lines corresponding to both these sub-types have been cultivated separately in the lab for decades.
Using engineering technologies, Rivron and his team assembled them for the first time in such a way as to trigger self-organisation, resulting in the formation of what they called "blastoids".
Chatty stem cells
"In a natural embryo, those same stem cells are in three dimensions talking to each other in a language that we barely understand," Rivron said.
The experiments mimicked that process, and the cells spontaneously began to arrange themselves as they might in the womb.
"The embryonic cells were the chatty ones here—they are instructing the placental stem cells to multiply, organise and implant into the uterus."
The findings could shed light on adult conditions that originate from small flaws in the embryo, including some forms of diabetes or cardiovascular disease, the study said.
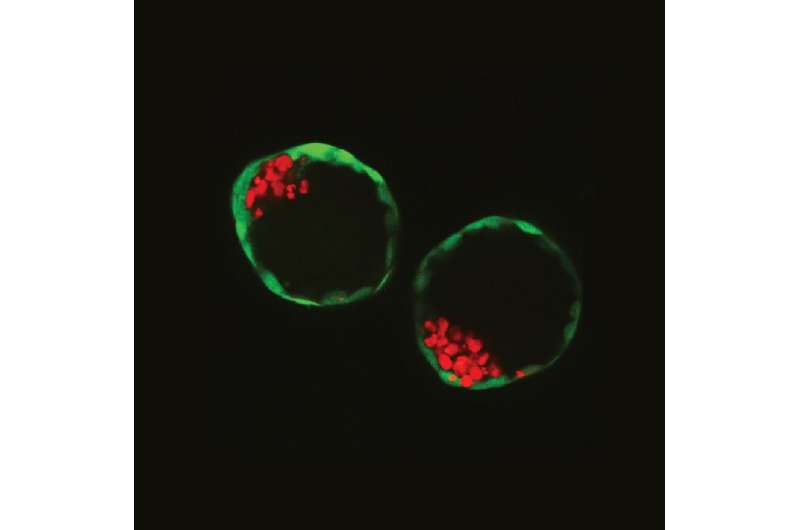
"This research opens the path to a new biomedical discipline," said co-author Clemens van Blitterswijk, a pioneer in tissue engineering and regenerative medicine at Maastricht University.
"We can create large numbers of model embryos and build up new knowledge by systematically testing new techniques and potential medicines."
It also dramatically reduces the need for animal experimentation, he added.
Outside experts hailed the results.
"These findings may help us to understand more about some aspects of infertility and improve outcomes of assisted reproduction," commented Dusk Ilic, a reader in stem cell science at King's College London.
Harry Leith, Group Head at MRC London Institute of Medical Sciences, acknowledged the breakthrough, but cautioned that it was unlikely to be duplicated with human stem cells anytime soon.
The experiments "appears to be the most successful attempt so far reported to 'build' an early embryo exclusively from cultured stem cell lines," he said in a comment provided by the Media Science Centre.
"However, we have yet to produce human stem cell lines with properties similar to the mouse cells used in this study.
More information: Blastocyst-like structures generated solely from stem cells, Nature (2018). nature.com/articles/doi:10.1038/s41586-018-0051-0
Journal information: Nature
© 2018 AFP