Professor Tom Solomon was in the shower when he got a sinking feeling in his stomach. It was July 2014 and Radio 4’s Today programme was reporting on Ebola. As director of the University of Liverpool’s Institute of Infection and Global Health, he was already well acquainted with the outbreak, which had been first reported in March, in Guinea. But there had been outbreaks of Ebola every few years in Africa since the mid-70s, and they were normally brought under control fairly quickly. True, this outbreak looked bigger than on previous occasions – there had already been a few hundred deaths – but Solomon put this down to the fact that it was the first time Ebola had hit west rather than central Africa, and people were unprepared.
That morning, Solomon heard two things that gave him pause. First, the doctor who was leading the response in Sierra Leone, Sheik Humarr Khan, had caught Ebola himself; he later died. And second, for the first time, an airline passenger had unwittingly carried Ebola to a new country, Nigeria.
By now Solomon’s mind was racing. If Khan had caught the disease, and he was meant to be leading the response, there was no way this outbreak was going to be controlled without much more help from outside. And the fact that an airline passenger had carried Ebola was also highly significant: this was the first time it had happened. If Ebola could be carried on a plane to Nigeria, where next?
He’d had that sinking feeling twice before in his life – when he didn’t think he’d be able to escape from a hotel fire in Vietnam, and as a junior doctor, when a patient was bleeding out in front of him. He knew something needed to be done quickly. If it wasn’t, the consequences would be disastrous.
Three months earlier, in April 2014, the institute had won a £4m grant to open a new health protection research unit specialising in emerging infections. As part of the application, Solomon had gone into great detail about any number of dangerous infections – Japanese encephalitis, tick encephalitis, influenza, dengue, West Nile virus, enterovirus 71, hantavirus, hepatitis E, Lyme disease. Did he mention Ebola? Solomon smiles. “Yes. Ebola was a footnote in our application… Little did we know.”
Now, Solomon and his team immediately got to work. By August, the World Health Organisation declared the epidemic to be an international public health emergency – the largest outbreak in history. It was a disease with an appalling mortality rate, estimated at 70% by the WHO last October. Official figures record that almost 10,000 people in west Africa have died so far; unofficially, that figure is feared to be far higher. And although the numbers of cases appear to be coming under control, they could go up again.
The institute’s new health protection research unit expected to divide its £4m among the many infections it has been researching over the years. Not surprisingly, things haven’t worked out like that. “Much of our activity has been diverted to Ebola,” Solomon says. For the infectious diseases industry, the pace of research has been astonishing. It often takes decades to develop vaccines against deadly infections, and some of the deadliest bugs, such as malaria, are still taunting the world’s top scientists more than a century after being discovered. “As a community, we have shown that when we need to set up drug and vaccine trials quickly, we can respond,” Solomon says. “And we hopefully will have a vaccine and treatment at the end of this terrible disaster, so some good will have come out of it.” His team is supporting several studies, including one led by his colleague Dr Peter Horby in Oxford, and another led by Belgian scientists.
The first thing Solomon’s team assessed was where the disease would spread next. They predicted that the US would be the first non-African country to import Ebola, which it did in September, and, with eerie accuracy, that Britain would have one case by the end of 2014.
Perhaps the most astonishing thing about Ebola is that, despite it first being identified in the 1970s, and despite regular outbreaks, so little work has been done until now to combat it – no effective drugs, no vaccine, just the standard procedures of isolating patients, not touching them, regular dousing in chlorine bleach for the uninfected.
The primary source, where the Ebola virus hangs out naturally, is still not completely clear. It has long been thought to circulate in fruit bats, which then pass it to other animals including monkeys and antelopes. Humans can become infected from the bats directly, or from eating bushmeat. The first known patient in the current outbreak, two-year-old Emile Ouamouno, is thought to have caught the disease from playing with infected fruit bats in a hollow tree in Meliandou, a tiny village deep in the Guinean forest. It was then transmitted to others via a classic, deadly pattern: through human-to-human contact, often during preparation for funerals, when families wash their loved ones’ bodies. At the point when Ebola victims die, they are at their most infectious. There were only 31 houses in Meliandou, but within four months of Emile’s death, 13 others in the village had been buried.
The WHO reports that the spread of the disease has now slowed, from a peak of hundreds of new cases a week to less than 100 cases a week in late January, but by the beginning of March had risen to 132 – 81 of them in Sierra Leone. A vaccine is being trialled and earlier predictions of 1 million people being infected will hopefully prove to have been an unhappy blip for the statisticians.
But whatever you do, Solomon says, don’t underestimate how serious the epidemic has been – and continues to be. “It’s been an emergency, a crisis, a catastrophe, all of those things. To have 10,000 deaths… it’s been a disaster.”

The institute’s base, the Ronald Ross Building, is in the centre of a building site in downtown Liverpool. At a glance, it’s hard to know whether the buildings are going up or coming down. In fact, this is one of the city’s growth areas, known as the Knowledge Quarter. Biomedical research is booming here. More than 300 people work in the institute – medics, scientists, managers. Every room is a melting pot of accents and nationalities. Solomon prides himself on recruiting from far and wide: if you want the best, he says, you’ve got to go global.
When the institute was formed in 2010, it brought together medics, vets and scientists working on infection and global health problems. “We’re probably the only UK institute that has these groups working side-by-side at the bench,” Solomon says. “Zoonotic bugs – those that jump from animals to humans – are becoming more and more important. So the One Health approach, which recognises that the health of humans, animals and the environment are interconnected, is key.” Another thing that makes the institute unusual is its gender balance: almost 50% of the scientists here are women. (Recent statistics show that in the UK only one-third of science graduates are female, and just 9% of professors.)
Britain, France and the US have led the way in tackling Ebola – and the institute is one of Britain’s leading research units working on the virus, alongside Public Health England, Oxford University, Imperial College London, and the two schools of tropical medicine in London and Liverpool. Liverpool has been at the heart of research into infectious diseases for more than 100 years. As one of the country’s leading port cities, it did extensive trade with Africa and Asia, and as a result attracted more than its share of tropical diseases. The Liverpool School of Tropical Medicine was founded in 1898 by Sir Alfred Lewis Jones, a local shipowner, a year before its London counterpart. It was the first institution in the world to specialise in teaching and researching tropical diseases. And the city’s own Ronald Ross was the first British winner of the Nobel prize in physiology or medicine, for showing that mosquitoes transmit malaria.
Ross is one of Solomon’s heroes. A cabinet on the ground floor of the institute contains his antique microscope, the one he took into the field in India. “There are not a lot of positives about mosquitoes,” Solomon says. “When people say what animal is the biggest killer of man, some say sharks, some tigers, but it’s actually mosquitoes. They kill through malaria, they kill through encephalitis, they kill through dengue. Some other insects such as ticks and midges also carry bugs, but mosquitoes are far and away the nastiest.”
I first met Solomon in 2013, at a fundraising function for the Encephalitis Society. We are both ambassadors (he because he is one of the country’s top experts on this infection of the brain, me because I survived it). In a brightly coloured waistcoat and oversized spectacles, he had something of the children’s TV presenter about him. He addressed a room of City bankers who knew nothing of the disease. Solomon captivated his audience – he was clear, accessible, moving and, at times, even amusing about this often potentially fatal brain infection. Next time I heard of him, he was presenting a video to publicise encephalitis in which he broke a Guinness world record for the biggest organ made of people: a human brain composed of hundreds of people in multicoloured cagoules.

Solomon grew up in Manchester to an accountant father and teacher mother. He was bright enough as a schoolboy, but nothing special. Ditto university, he says. It was only when he discovered bugs that he came into his own.
It’s not easy to stop Solomon, 49, when he’s talking about infections. This isn’t a job, it’s a vocation. Possibly an obsession. It all goes back to his first trip to Africa as a medical student. He had thought he would be a GP until he started working with malaria patients. “Occasionally, you’ll sit in a clinic in this country and think some of these people haven’t got that much wrong with them, but it wasn’t like that out there. There were massive challenges that you could do something about if you were lucky.”
He had planned to conduct a study to see if malaria patients had low blood sugar. However, just as he was heading to Mozambique, someone published a paper in the Lancet showing exactly that. So he broadened his research question to look at the relationship between blood sugar levels and all serious childhood diseases. He tested every child who presented, through day and night, to the point of being ill himself. He discovered that many sick children had low blood sugar levels, not just those with malaria, and his findings made it into the Lancet, too.
Solomon returned from Africa, underwent clinical training and then, in his late 20s, headed to Vietnam with his girlfriend Rachel, to work on the Japanese encephalitis epidemic. Three years later, he returned to Britain with Rachel, by now his wife, and two adopted Vietnamese babies. Leah, now 19, was brought to the hospital with tetanus when she was 10 days old. Solomon and Rachel adopted her. Just before they left Vietnam, baby Daisy was brought to a local orphanage and they adopted her, too. They also have two younger biological daughters, Rosie and Eva – they’re “home-grown,” Solomon says.
Solomon’s office is a testament to his many passions. On his desk is a glass kukri full of rum, a gift from his research fellow in Nepal. “I’ll only give you a little bit because it’s very strong,” he says, “but at least you get a flavour.” He pours and clinks glasses. “Cheers. I don’t normally drink it at work.”

On his cabinet is a picture of Rachel and the girls, behind his chair is a signed Liverpool football shirt, and balanced precariously on a high shelf is an Olympic torch (he was an official torchbearer in 2012). His bookshelves are crammed with medical textbooks, a number of which he has written or edited. He shows me a certificate of which he is particularly proud, stating that: “The Guinness world record for the fastest marathon dressed as a doctor was achieved by Tom Solomon in four hours 21 minutes and nine seconds.” He ran as The Running Mad Professor (his Twitter handle is @RunningMadProf), raising £21,000 for the Encephalitis Society.
On the wall are framed photographs of heroes from history. “Sir William Osler is the man who essentially defined medical science as we’re still doing it 150 years later. He was one of the guys who said: ‘We need to study diseases by looking at people who are sick, at the bedside.’ Before that, a lot of it was just theory, pure fancy, learned in the lecture theatres.” He points to another photograph. “That’s Alexandre Yersin, who was in Vietnam. He discovered that plague was caused by a bacteria. I was in Vietnam 100 years after him. Then, not long after, we had Sars happening in south-east Asia, and there were a lot of parallels.”
Solomon is a great fan of practitioner-scientists such as Osler and Yersin. These days, he spends two days a week working with patients at the Walton Centre and Royal Liverpool University hospital, and three at the institute. For the past 20 years, he has specialised in encephalitis – another infection with a shocking mortality rate. Encephalitis is an inflammation of the brain that, in Britain, is often caused by the herpes virus (unlike Japanese encephalitis, which is passed on through mosquitoes). Survivors of encephalitis are often left with complex, untreatable problems. Solomon tells me of a patient who cannot lay down new memories. “He can’t remember anything since the 1970s. His hard drive, if you like, has lost 30 years’ worth of memories.”
We head for lunch in the Victoria Gallery & Museum, the original Liverpool University building. “Just think,” Solomon says, “the building next to us is where Ronald Ross worked, and 100 years on we’re still working on emerging diseases spread by mosquitoes.” A sit-down lunch is a luxury – he normally grabs a sandwich. Over lunch, it becomes apparent that he has quite an affection for infections. He anthropomorphises viruses, tells me why we have to look at things from their point of view rather than from ours – they are just trying to get on with life, reproduce and survive. People assume that they want to kill humans, but the truth couldn’t be more different. Those viruses that know what is good for them may make people ill or lie latent for years, surviving off humans, but not destroying us. After all, if they kill us, they kill themselves.

Back at the institute, Chris Moxon, a scientist in his 30s, is sitting at his computer, studying cerebral malaria. Three years ago he was in Malawi working with malaria patients. Now he’s applied for a £600,000 grant to further his research. Does he hope to find a solution to the disease? Moxon, a quietly spoken intellectual, is amused by the question. “You can’t apply for a grant saying you’re going to solve cerebral malaria, because people will laugh.”
Solomon: “It’s all incremental.”
Moxon: “Yes, you just chip away.”
Solomon: “When I was a youngster, there were 2 million deaths a year from malaria. Now it’s half a million deaths a year.”
Moxon is not entirely convinced. “It depends on who you go with. Chris Murray [a researcher in public health and global health at the University of Washington] said [there were] 1.2 million deaths a year in 2012. He thought the WHO underestimated it.”
While Moxon and Solomon quibble over figures, they agree that Ebola is far more likely to be cured than malaria. “The difference with Ebola is that, potentially, within a short cycle, by the end of 2015, we might well have a treatment that works and a vaccine,” Solomon says. A team led by Professor Julian Hiscox at the institute, in collaboration with Public Health England, has been looking for possible treatments. They examined which proteins inside a cell are hijacked by the virus to help it reproduce, and discovered that one virus protein, known as VP24, disrupts signalling in infected human cells and so impairs the fight against the virus. In September, Hiscox suggested that a drug known as ouabain, traditionally used for severe heart disease, could reduce the virus’s replication.
Is Moxon surprised by the pace of progress with Ebola? “It is extraordinary. It is wonderful to have the huge world interest and everybody coming together. But even with that amount of money and attention, you couldn’t make that amount of headway on malaria.” You get the impression that neither Solomon nor Moxon much respects Ebola as a virus, whereas malaria is held in awe.
Malaria is simply smarter, Solomon says. “It has been learning how to avoid our immune system for millennia, because we are the natural host for it. I remember when I was an even younger student than you,” he tells Moxon, “in Mozambique, we went to a village and bled all the kids there. They were playing football and having fun, yet one in three of them had malaria parasites in their blood. So this parasite has learned how to live in the human body without making you so sick that you die, because it doesn’t want to kill you. You’ve got to look at it from the parasite’s viewpoint; it just wants to hang out in there.”
“And occasionally, unfortunately, it makes someone really sick,” Moxon adds.
“Don’t get me wrong,” Solomon says, “Ebola isn’t stupid – in fact, it’s pretty good at duping the immune system.” But malaria is more sophisticated in that it can infect the host again and again. Ebola either kills the human or is killed by the human. “Millions of people get malaria. The only people who get Ebola are those who are caring for somebody who is ill with Ebola. Unless you’re mopping up after them, you’re not so much at risk.”
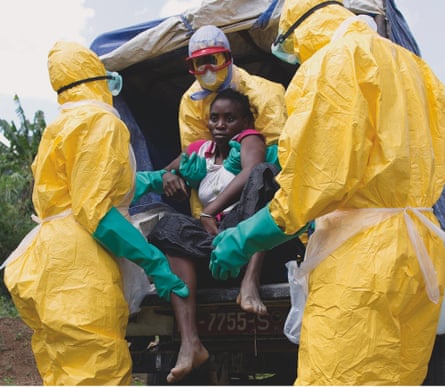
The problem in west Africa is that so many of the medics were infected. “The healthcare services there are falling apart. In some places, half the doctors and nurses are dead.” For this reason, Solomon says, it is vital that UK healthcare workers continue going to west Africa to diagnose and treat patients. He mentions the nurse Pauline Cafferkey, who was diagnosed only once she had returned to Britain, acknowledging that her story is enough to put some off going out to help. “She was protected, working for Save the Children, she shouldn’t have got sick. And if someone like that is getting ill, it makes people think, blimey. The original west African doctors and nurses who died, it was because they didn’t have proper gear to protect themselves. If Pauline Cafferkey had died, it would have a bad impact on people’s willingness to go. But, in some ways, this isn’t new: medics have always been at risk of infectious diseases. A lot of doctors used to die of TB just because they were exposed to it.”
Thankfully, Cafferkey made a good recovery, but does he feel anxious when his staff go to west Africa? Yes, of course, he says – and slightly guilty. “If I’ve got people going out there, obviously I’d like to think I’d lead by example.” Solomon has volunteered to go, but it has been decided that he is needed more at the institute, running things there.
Is there any chance of an Ebola epidemic in Britain? No, he says, this is when the NHS comes into its own. “The government can say, ‘This is the kind of hospital that can take patients, this is how they should be set up’, whereas in America it’s a series of states that do their own thing, so patients turned up and they were not prepared for it.”
Twenty-six-year-old biotechnologist Raquel Medialdea Carrera has been at the institute for four months and is about to fly out to Sierra Leone, where she will be working in the diagnostics laboratory set up by Public Health England. Is she nervous? “Not yet. I got very nervous when I had to tell my parents, who are back in Spain. That was scary. First I told my father, and he said don’t tell your mother until you’re there. But I told her. And they understood.”
What do her friends think when she tells them she is going? “Most of them are like, ‘Are you crazy?’ The second reaction is, ‘Don’t get close to me for a few months after you’re back.’”
Medialdea Carrera will be in Sierra Leone for five weeks – the maximum stint allowed. She will wear a full body suit to work, and is allowed to do only three one-hour shifts a day. “Because it’s absolutely exhausting,” Solomon explains. “It’s like stepping into an oven dressed in this plastic suit.”
When she returns, she will get time off work to ensure she’s healthy and to readjust. It is not uncommon for people to return traumatised after what they’ve seen. The horror is not just in the number of deaths, but in their manner.
We’re heading towards the containment level three lab. This is the highest containment level at the institute (the absolute highest is containment level four, run by Public Health England at Porton Down – full body suits are needed even to enter, and that is where the actual Ebola virus is worked on). To enter containment level three, we have to pass through the kind of air-locked doors you would find in a high-security prison. The room is freezing, and the only noise is the constant mechanical whirring of the negative pressure cabinet that pulls in the air flow, so if there is a spillage of virus, it will rise up through the filters and keep workers safe. Solomon points to a massive freezer regulated at -77C to keep all the viruses in peak condition (home freezers work at -20C). On the door of the freezer are the names of all the viruses deposited (dengue, Japanese encephalitis, chikungunya) and the scientists who have been working on them (Lucy, Daniel, Denis, Jenny, Neil and Kevin). The casual intimacy of the first names next to the deadly disease labels is unnerving.
Of all the diseases Solomon has worked with, it’s Japanese encephalitis that continues to fascinate him most. Another clever bug, he says, and one that he has enjoyed most success combating.
When he went to Vietnam in 1991, the children who were unwell, in comas or having fits, were thought to have cerebral malaria, but it turned out to be Japanese encephalitis. Others looked as if they had polio, even though polio had been pretty well eradicated. These children also proved to be infected with the Japanese encephalitis virus. The disease manifests itself in different forms, making it tricky to diagnose. Twenty years ago, there was only one international lab in south-east Asia where they could test for it, so Solomon and his colleagues at the University of Malaysia in Sarawak developed a new diagnostic kit: a simple test they could do by the bedside.
“We call it a dot blot kit,” Solomon says. “Essentially, you were putting blood on blotting paper and looking at the colour change. Tests like that are now used across the whole of south-east Asia.” They also devised a simple way of measuring the outcome of Japanese encephalitis, now known as the Liverpool Outcome Score. This score means that governments can work out how much disability there is, how much Japanese encephalitis is costing them, and decide whether it is cost-effective to vaccinate. And most have decided it is. The end result is that manufacturers have brought down the cost of the vaccine and are producing millions of doses.
But Japanese encephalitis remains incredibly difficult to treat. While it is estimated that over the past 10 years up to 800,000 case have been prevented through the use of vaccines, there is still no drug to treat those who get infected. In one trial, interferon, a drug traditionally used for cancers and hepatitis, slowed down the illness, but did not stop people dying. Solomon and his team, the Brain Infections Group, are still working on other treatments.
Meanwhile, the virus is spreading to new geographical areas. The last decade has seen the disease creep up towards the highlands of Nepal. Previously, it was found only in the low-lying rice paddy areas. “We’ve been tracking it,” Solomon says, “and one possibility is that climate change is allowing the disease to spread.”
Japanese encephalitis, which is carried by mosquitoes, is unlikely to become a problem in the UK, but Solomon wouldn’t rule anything out. “With climate change, some diseases that couldn’t come here because it was too cold might be able to in the future. Dengue is another disease carried by mosquitoes that we’re seeing in Europe a bit, and that might come to the UK eventually. There is also lots of tick-born encephalitis in Europe, and the UK is at risk from that, too. So it’s about looking at what the risks are and trying to mitigate them.”
So what is the greatest threat facing people in the UK? Ah, Solomon says, the biggest threat is not a single disease. He takes me down to a huge laboratory to explain. This is only containment level two, meaning we can walk around in white coats, but the work going on here is vital for our future. “There was a time when we thought we had infections cracked – we could just give antibiotics for everything.” That is, until we started to overuse them, both in treating human infections, but also in the farming industry.
Now, antimicrobial resistance, also known as antibiotic resistance, is one of our greatest threats. “Bugs are becoming resistant to antimicrobials,” Solomon says. “It’s just Darwinian evolution.” The rise of these antimicrobial-resistant bugs is a terrifying prospect, and that is why places such as the institute are dedicating so much energy to tackling it.
We meet Elaine Waters, who is counting bacteria in a small dish to see how many are resistant. She is part of a team led by Aras Kadioglu looking for alternatives to antibiotics. Their findings have just been published in the science journal Nature, showing a new way of treating bacterial infections. Instead of antibiotics, liposomes – tiny, lab-produced bubbles of fat – are used to vacuum up the toxins.
These liposomes could be lifesavers for millions. But, as with most medical research, we are talking long-term, Solomon says. “The next step is to show it works in animals, then to show it’s safe in humans. The clinical trials take years to set up and do, which is why the Ebola thing is so remarkable: it’s all been done over months.”
Meanwhile, to control antimicrobial resistance, we have to make better use of the antibiotics we have. “We’ve got to somehow encourage GPs and the public to reserve them for those who are really unwell. Convince farmers globally not to use them ad lib to make animals grow more quickly.”
Solomon hopes that during 2015 scientists will get the better of Ebola. And then they will focus on the many other perils, known and unknown, that follow in its wake. Yes, there may be other potential Ebolas out there, but one of the most important battles to be fought in the world’s laboratories over the next few years will be antimicrobial resistance. If antibiotics do fail us, it could be catastrophic for mankind – pneumonia, for example, would once again become a common killer.
But Solomon is an optimist: rather than focusing on the threat, he prefers to see it as an opportunity. He looks at the liposomes dancing in their tiny dish of bacteria and toxins rather lovingly: “In 10 years’ time, if you’re unfortunate enough to get a severe bacterial infection, you may well be cured with the treatment that has come from this institute.”

Comments (…)
Sign in or create your Guardian account to join the discussion