Renewable energy generation does not always meet demand. This is for the simple reason that consumers may wish to use energy when the sun isn’t shining or the wind isn’t blowing. Batteries can be used to balance this generation and demand problem. Batteries can charge when energy is generated and discharge when energy is needed.
Batteries can also do a lot more than simply balance energy generation and demand. Batteries can assist with a vast range of grid applications including back-up services, generating synthetic inertia and converting non-synchronous power to synchronous power. These are all services that are essential for the reliable operation of an electricity grid.
So how do batteries work?
Batteries have three key parts:
- a positive electrode;
- a negative electrode; and
- an electrolyte which separates the electrodes.
Different electrodes and electrolytes produce different chemical reactions that affect how the battery works. This is why there is a large diversity of batteries on the market and in development. Different battery characteristics include:
- size;
- storage capacity;
- response rate;
- recharge rate;
- lifespan;
- safety;
- costs; and
- recyclability.
The vanadium redox battery (VRB) is one of the latest batteries to emerge on the commercial market. It is particularly promising because of its extremely large storage capacity. From an environmental perspective, the VRB is also promising. The VRB does not produce waste products and has a long life span. The chemicals in a VRB do not need to be replaced and can be used endlessly. Only the VRB housing and hardware will need to be replaced. The long life span also means that the VRB has the potential to be a low cost alternative to other options.
The VRB has a positive and negative chamber separated by a membrane. The positive chamber has a positive electrode and the negative chamber has a negative electrode. Vanadium electrolytes circulate in both chambers: - a positive vanadium electrolyte or species in the positive chamber, and a negative species in the negative chamber. The chambers are connected to storage tanks so that large volumes of the vanadium electrolytes can be pumped in and circulated throughout the chambers. The membrane stops the positive and negative electrolytes from mixing, but allows ions (atoms with a positive or negative charge) to pass through.
When the VRB is charged and discharged the vanadium species simultaneously undergo oxidation and reduction, transferring electrons through the membrane. The oxidation-reduction reaction is known as a redox reaction. Reduction involves gaining electrons. Oxidation involves losing electrons. During charging the electrolyte in the positive chamber is oxidised, and the electrolyte in the negative chamber is reduced. During discharge the process is reversed, and the electrolyte in the positive chamber is reduced while the electrolyte in the negative chamber is oxidised.
The charge redox reaction can be used to store electricity when it is generated and the discharge redox reaction can supply electricity when it is demanded. The VRB has a 75% - 80% charge / discharge efficiency.
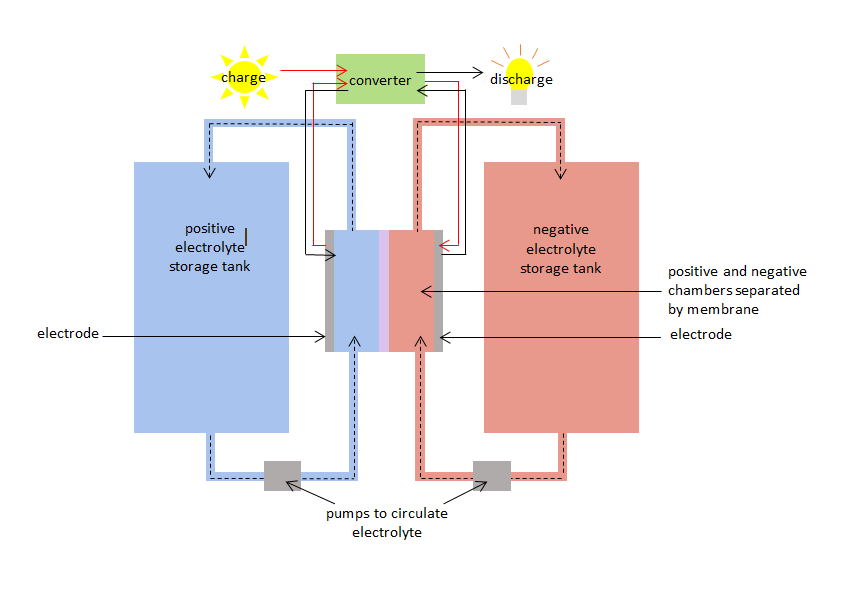
Diagram of vanadium redox battery



