Introduction
In most European countries the numbers of farmland birds have declined over recent decades (Chamberlain et al. Reference Chamberlain, Fuller, Bunce, Duckworth and Shrubb2000, Donald et al. Reference Donald, Green and Heath2001, Reference Donald, Sanderson, Burfield and Bommel2006, Chamberlain and Vickery Reference Chamberlain and Vickery2002, Butler et al. Reference Butler, Boccaccio, Gregory, Vorisek and Norris2010, PECBMS 2013). There is an increasing evidence that one of the main problems for ground-nesting birds is low breeding success due to intensive agriculture and predation (e.g. MacDonald and Bolton Reference MacDonald and Bolton2008, Roodbergen et al. Reference Roodbergen, van der Werf and Hötker2012). Several approaches to the elimination of nest destruction and depredation have been developed in many European countries, including various forms of direct nest protection (Guldemond et al. Reference Guldemond, Parmentier and Visbeen1993, Isaksson et al. Reference Isaksson, Wallander and Larsson2007, Kragten et al. Reference Kragten, Nagel and De Snoo2008, Grüebler et al. Reference Grüebler, Schuler, Horch and Spaar2012, Kentie et al. Reference Kentie, Both, Hooijmeijer and Piersma2015, Santangeli et al. Reference Santangeli, Arroyo, Millon and Bretagnolle2015, Sutherland et al. Reference Sutherland, Dicks, Ockendon and Smith2015). On meadows and arable land, the most widely-used technique is conspicuous marking to make the nest site visible to farmers operating machinery, e.g. with bamboo poles (Kragten et al. Reference Kragten, Nagel and De Snoo2008, Schifferli et al. Reference Schifferli, Spaar and Koller2006, Reference Schifferli, Rickenbach, Koller and Grüebler2009). Farmers usually drive round the nest and leave a small part of the land undisturbed. The area of undisturbed land varies from several square metres in the case of waders and songbirds (Kentie et al. Reference Kentie, Both, Hooijmeijer and Piersma2015, Schifferli et al. Reference Schifferli, Spaar and Koller2006; Grüebler et al. Reference Grüebler, Schuler, Horch and Spaar2012) up to dozens of square metres in the case of Montagu’s Harrier Circus pygargus (Kunstmüller and Kodet Reference Kunstmüller and Kodet2008). Direct protection is primarily applied to avoid nest destruction by farm machinery, but the use of relatively short poles just 1 m in height and inconspicuously coloured may not be sufficiently visible to farmers, and may therefore not be very effective in nest protection (Kragten et al. Reference Kragten, Nagel and De Snoo2008). At the same time, marking itself has been considered to increase the risk of nest depredation (Kragten et al. Reference Kragten, Nagel and De Snoo2008). However, the assumption about the risk of depredation of directly protected wader nests has never been properly verified experimentally.
The objective of our study was to investigate the use of long poles that are more visible to farmers and therefore more effective for direct protection of nests. It provides new findings from the Czech Republic, where the local population of Northern Lapwing Vanellus vanellus dropped by around 90% between 1982 and 2015 (Czech Society for Ornithology 2015). Most of this population breeds on arable land, where it is strongly dependent on farmland practices. As in other European countries, the main factors responsible for this decline are intensification of farming which includes irrigation, conversion of grasslands to arable, the development of agricultural machinery, increased use of pesticides and fertilisers (Fiala Reference Fiala2002, Šťastný et al. Reference Šťastný, Bejček and Hudec2006, Kubelka et al. Reference Kubelka, Zámečník and Šálek2012a, Zámečník Reference Zámečník2013), and predation of nests and chicks (Šálek Reference Šálek2000). On grasslands, the most high-risk operations are spring rolling and harrowing (Šálek Reference Šálek2000, Kubelka et al. Reference Kubelka, Zámečník and Šálek2012b); on arable land, the nests are often destroyed during cultivation of ploughed and fallow fields and when spring crops are sown (Kubelka et al. Reference Kubelka, Zámečník and Šálek2012a). Since 2009, direct protection of Lapwing nests has been one of the cross-compliance requirements. All farmers in the Czech Republic receiving direct payments are obliged to avoid destruction of nests when they have been officially informed about their position (Ministry of Agriculture of the Czech Republic 2015). This tool is still implemented only occasionally, but on traditional breeding sites it can be a crucial way of eliminating the destruction of clutches by farming activities. However, before this option can be promoted more widely among volunteers it is necessary to gather enough evidence that it is an effective measure and constitutes best practice. For this reason, the main objective of our study was to test experimentally whether marking the nests with two thin bamboo poles which would be visible enough to operating farmers affects the risk of predation on active Northern Lapwing nests. Our study aimed to provide evidence on whether nest marking of this type can be considered a safe conservation tool as regards the nest predation risk to ground-nesting birds in an agricultural landscape.
Methods
Data collection
Field work was carried out between 2010 and 2013 in two regions of the Czech Republic, one in South Bohemia (49.12N, 14.31E) and one in East Bohemia (50.18N, 15.61E), with a total area of about 500 km2. In both regions, the dominant habitat is agricultural land, mainly a mosaic of arable (winter wheat, ploughed fields, spring cereal, oilseed rape, maize) interspersed with meadows, pastures (only in south Bohemia), linear non-cropped habitats along ditches and roads and, especially in south Bohemia, fishponds. The main potential nest predator species (red fox Vulpes vulpes, beech marten Martes foina, pine marten M. martes, stoat Mustela erminea, weasel M. nivalis, European hedgehog Erinaceus europaeus, Marsh Harrier Circus aeruginosus and Carrion Crow Corvus corone) are identical for these two areas (own observations and data from cameras placed at the nests).
Northern Lapwing breeding sites were determined on the basis of the conspicuous display and courtship behaviour of birds (e.g. Cramp and Simmons Reference Cramp and Simmons1983) from the second half of March until the end of May. Nests were located either visually with the use of binoculars and spotting scopes, or by direct inspection of densely populated fields by a skirmish line with 5–8 (max. 12) observers (Kubelka et al. Reference Kubelka, Zámečník and Šálek2012b). The positions of the nests that were found were stored in a GPS tracker. All nests were marked with a thin willow twig 50 cm long fixed 15 m from the nest, exactly as in Šálek and Šmilauer (Reference Šálek and Šmilauer2002). This inconspicuous marking was found not to affect nest survival (Galbraith Reference Galbraith1988). The incubation stage was assessed using a flotation test (van Paassen et al. Reference van Paassen, Veldman and Beintema1984). When more than one nest was found in the same type of habitat and with a similar incubation stage and position within the field, pairs of nests were established and one (randomly selected) of the nests was provided with bamboo poles. Paired nests were chosen to be approximately 50–200 m away from each other. The bamboo poles were 2 m in length, 2–3.5 cm thick at the base, and 1 cm thick at the top. The top end was highlighted with a reflective red or orange spray. The sprayed part of the bamboo was 15–20 cm in length. The bamboo poles were fixed along the line of cultivation 10–12 m apart, with a nest in the middle.
Our experiment was designed exclusively to test nest predation risk, i.e. nest pairs were situated in fields where no immediate farming activity was expected. Nevertheless, farmers were informed about the position of poles and if we were informed about an unexpected farming operation that could cause nest destruction, the experiment ended just before this operation (as control nests were also protected by bamboo poles). Both paired nests were repeatedly visited on the same day at irregular intervals, with a median of seven days (minimum two days and maximum 18 days), until the final fate of any of them was determined. Nests were recorded as successful when at least one egg hatched. Eggs were assumed to have hatched successfully when chicks or small remnants of eggshell were present in the nest (Green et al. Reference Green, Hawell and Johnson1987). Nests were assumed to have failed when no eggs hatched. If a nest was found empty, without eggshell remnants, or with large pieces of eggshell nearby, the nest was recorded as depredated. If there were signs of recent farming operations, and remnants of the nest were found, the nest was recorded as failed due to farming activities (three nests in two pairs, one nest even with bamboo poles). In our dataset, the losses were due only to predation and agricultural machinery; there was no desertion or other reason for failure. Once one of the nests was depredated or destroyed, the experiment on that pair was terminated. The date of predation was then calculated as the midpoint of the period between the last visit when eggs were present and the final visit. For the three nests (two pairs) destroyed by farm machinery, the experiment was terminated by the date of the last positive visit.
Data analysis
We used a paired t-test to test whether both marked and unmarked nests were equally distributed in respect to distance from the habitat edge. In order to assess whether the nests provided with poles also attracted predators toward the nest counterparts without poles, we compared the proportion of simultaneous predation events on both nests within nest pairs and proportion of predation events on just any one of the two nests within a pair. If the former prevails, we can assume significant attraction of poles for predators to both nests in a pair. The nest predation rate was calculated according to Mayfield (Reference Mayfield1975) as the proportion of the number of depredated nests and the sum of nest-day exposures. Hatching success reflected the daily survival rate powered by the mean incubation period of Northern Lapwing (27 days; Cramp and Simmons Reference Cramp and Simmons1983).
A mixed-effect model (GLMM) with the chi-square testing procedure (likelihood ratio test, LRT) was applied to assess the fixed effects of poles, incubation stage, habitat, distances from the habitat edge and the interactions of the poles with all remaining predictors on the nest predation risk (response variable) expressed binomially (surviving = 1, predation = 0). Non-predation means a still active nest with eggs, or a hatched nest. The nest-specific incubation stage on the day when the experiment began might add to the explanation of nest depredation, so we included it in the model. As the locality might pseudo-replicate the predation risk of the same predators, we assigned nest pairs and breeding grounds as random effects. First we tested the effects of interactions, and after they had been removed we checked the contributions of the fixed effects (Crawley Reference Crawley2007). We adopted α = 0.05 for the rejection of a hypothesis. We also checked the relationship between incubation stage on the day when the poles were installed and the day in the season (corrected by median date of incubation start in analysed nests within particular years). All statistical procedures were performed by ’lme4’ package in R, version 3.1.2 (R Core Development Team 2014).
Results
A total of 104 nests in 52 pairs of nests in 15 localities, accounting for 2004 nest-days of exposure and 57 depredated nests were included in the analysis (Table 1). The distance from the nearest habitat edge of nests provided with poles [140 m ± (SE) 12.3 m] did not differ significantly from the control nests without poles [131 m ± (SE) 13.7 m] (paired t-test, t51 = 1.4, P = 0.18). The incubation stage on the day of the beginning of the experiment was identical for the nests provided with poles [nine days ± (SE) 0.8 days] and for the nests without poles [nine days ± (SE) 0.8 days] (paired t-test, t51 = 0.2, P = 0.82). Incubation stage was not correlated with day in the season (Spearman’s rank correlation coefficient rs = -0.16, P = 0.10).
Table 1. Dataset of nest pairs collected for various habitats in two areas in Bohemia.

The total daily nest predation rate was 2.8% ± (SE) 0.37%. The daily predation rate was 2.8% ± (SE) 0.54% in the marked nests (n = 52) and 2.8% ± (SE) 0.51% in the unmarked nests (n = 52), i.e. the hatching success was 47.0% for the marked nests and 44.8% for the unmarked nests. The mixed-effect model did not detect an effect of poles on the predation risk of the experimental nests (Table 2). The incubation stage was the only significant fixed effect; it showed that fresh nests were more prone to predation risk than nests closer to hatching date. As shown in Figure 1, nests found in the halfway incubation stage (14 days) still had about a 60% chance of survival while the nests found earlier had markedly reduced survival. We did not detect significant effects of habitat, distance from field edge or any interaction on nest survival, with the exception of the interaction poles × stage. This suggested that there were different effects of incubation stage in nests provided with poles and in nests without poles. A post-hoc analysis indicates that the nests without poles were more prone to depredation in the early stages of incubation (GLMM; estimate = 0.04 ± (SE) 0.011, χ2 = 12.2, P < 0.001) than the nests provided with poles (GLMM; χ2 = 1.9, P = 0.17). The proportion of simultaneously depredated nest pairs (40.4%) was not significantly higher than the number of predation events on one (28.8%) of the two paired nests (test of proportions, χ21 = 1.1, P = 0.30). We suggest that the poles did not affect simultaneous attraction to both nests within experimental pairs.
Table 2. Results of a mixed-effect model explaining the effects of the factors on the predation risk for the experimental Northern Lapwing nests. Ordered according to decreasing χ2 values. A positive estimate means increasing survival.

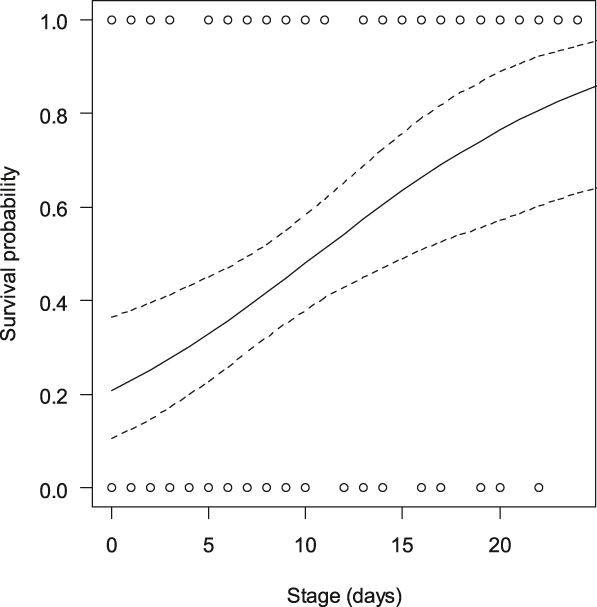
Figure 1. Probability (+95% CI) of nest survival (n = 104 nests) according to nest stage at the date of pole installation. All nests (provided with poles and without poles) are included.
Discussion
Although marking of ground-nesting birds’ nests for nest protection is generally used in many European countries, only a few studies have evaluated the effectiveness of this marking (Sutherland et al. Reference Sutherland, Dicks, Ockendon and Smith2015). Our experience indicates that, when applied in an optimal way, direct nest protection could be a suitable method for avoiding nest destruction during farming operations without raising the risk of nest depredation or desertion.
Probably the largest investigation was carried out in 2005 and 2006 in the Netherlands; this analysis included 1,644 protected nests against 229 nests without any protection (Kragten et al. Reference Kragten, Nagel and De Snoo2008). The authors recorded a higher rate of predation of the marked nests in one study area during one season. They admit that the conspicuous markings may enhance nest predation in some circumstances. In addition, 6% of protected nests were destroyed due to farming operations. According to Götmark (Reference Götmark1992), marking itself reduces nest destruction due to farming operations, but might attract predators through investigator disturbance. To avoid this potential bias, an experimental design based on pairing of nests, with only one of them marked and the other as a reference nest, was applied in our study. This design helped us to eliminate the effects of habitat, locality and to control the incubation stage at the date when the experiment started. However, our study has not revealed any impact of marking of nests on nest predation.
Timing of conservation action
Nests at earlier stages of incubation are under higher risk of predation as these include a group of poorly placed nests prone to be easily discovered by predators (Ricklefs Reference Ricklefs1969, Martin and Roper Reference Martin and Roper1988, Eggers et al. Reference Eggers, Griesser and Ekman2005). An explanation that fresh Lapwing clutches were defended less intensively and thus were more exposed to predation risk is not supported by previous investigation (Kis et al. Reference Kis, Liker and Szekely2000). If these early clutches are marked for a longer time before field cultivation, subsequent losses due to predation will make this measure inefficient due to the unreasonable demands that it makes on farmers as these either unnecessarily drive around depredated nest or have to stop the tractor to check the nest. If it is depredated, drivers have to take away the bamboo poles before continuing their work.
A further risk connected with marking of early clutches is nest desertion. In Switzerland, half of the Lapwing nests marked with bamboo poles while eggs were being laid were deserted, probably due to sensitivity of females to disturbance of this kind in the early stages of nesting (Schifferli et al. Reference Schifferli, Rickenbach, Koller and Grüebler2009). This was probably aggravated by the relatively close placement of the poles, only 2–3 m from the nest. Also Kragten et al. (Reference Kragten, Nagel and De Snoo2008) recorded greater desertion of marked nests than of unmarked nests. As the nests in their study were marked immediately after they were found, clutches in the early stages of incubation were very likely also included. In our study, nest marking did not result in any nest desertion, as bamboo poles were placed only when the clutches were complete. This indicates that clutches that are just being laid should be marked with poles only if field operations are imminent. If this is not possible, it is questionable whether the nests should be protected at all, having in mind the uncertain benefits of this measure in this particular case. Our finding that nests without poles were more prone to depredation in the early incubation stages than nests provided with poles we interpret as a type I error.
Optimal use of bamboo poles
In our experiment, poles were placed at least 5 m from the nest and there was no evidence of nest desertion. In previous studies, the poles were significantly closer (2–3 m in Switzerland, and 3–5 m in the Netherlands) and, as mentioned above, cases of nest desertion were relatively numerous. From the farmer’s point of view, it makes practically no economic or technical difference whether the poles are placed 3 m or 5 m from the nest. Therefore to eliminate possible disturbance to the birds, poles should be placed at least 5 m from the nest.
In addition, it seems that taller poles that are sprayed with a bright colour at the top end are more effective than shorter poles with a natural colour. Altogether with this project, from 2010 until 2016 we used direct protection for more than 400 nests and all cases of nest destruction (up to 4% of protected nests) were due to a communication failure (own unpubl. data). It is therefore crucial to stay in close contact with farmers. They need to be informed without delay, and must be given precise information about the number of nests, the way in which they are marked and the dates of hatching. It is also useful to provide a map with the positions of the nests. It seems that the use of a bright reflective colour at the top of poles acts optimally for informed farmers, even if they are working at night (own unpubl. data), and that the bright reflective colour does not attract potential nest predators.
Direct protection has also been used with success for protecting a small number of nests of rarer waders breeding in the Czech agricultural landscape in the South Bohemian region (own unpubl. data) – several tens of nests of Little Ringed Plover Charadrius dubius, three nests of Black-tailed Godwit Limosa limosa, and one nest of Redshank Tringa totanus. These species easily accept marking of their nests with bamboo poles, and direct nest protection was highly successful.
Disadvantages of direct protection
Although our results did not show an increased rate of predation due to conspicuous nest marking to inform farmers, there is still a question of the learning abilities of some predators. It has already been confirmed that some predators are able to remember the position of an incubating individual, and they visit the breeding site when the parents are away (Šálek and Zámečník Reference Šálek and Zámečník2014). Corvids, in particular, are known to develop their predation tactics and to learn. Once these birds connect poles with possible prey, marking could lead to increased predation. Another risk arises with the possible attractiveness of the small plots around the nest that are created as a result of the tractor driver’s efforts to avoid destroying a nest. This effect has already been proved for Montagu’s Harrier (Koks and Visser Reference Koks and Visser2000, Santangeli et al. Reference Santangeli, Arroyo, Millon and Bretagnolle2015). To provide evidence of this, however, further specifically designed experiment is required.
In addition, it is not known how predators would respond to a high concentration of poles installed near to the nests in large breeding colonies concentrated around one hotspot (e.g. a piece of waterlogged land inside an arable field). We suggest that it would be more effective and technically more feasible in this case to protect the whole nesting colony from the risk of damage by farmers, rather than marking and avoiding each nest individually. In the long term, the best option is to adopt targeted agri-environment measures that would create an optimal breeding habitat and would prohibit any agricultural activity during the breeding season. However, a measure of this type should preferably be applied at regular breeding sites of local importance, and only if allowed by legislation and accepted by farmers.
Conclusion
Our results show that it is possible to find a finely tuned trade-off in marking the nests of ground-nesting birds between the risk of damage by agriculture machinery and the risk of increased nest predation. Two thin bamboo poles with the nest located between them are sufficiently visible for the farmer but, at the same time, they do not attract potential predators. Our positive experience with Northern Lapwing and episodically with three other wader species in a mosaic of arable plots and meadows in the Czech Republic suggests that this type of direct nest protection could be used effectively for a wider variety of ground-nesting birds. However, it is necessary to carry out further research on the responses of individual species to this kind of disturbance in association with depredation risk in larger colonies. Although direct nest protection can be used as a suitable protection tool, it is time-demanding and should be applied only when other conservation measures fail. Especially for regular breeding sites, it cannot effectively substitute a targeted large-scale conservation measure, e.g. an agri-environmental scheme.
Acknowledgements
This study could not have been carried out without the help of many volunteers participating in the search for nests. Our special thanks go to Vladimír Štorek for his help with data collection, and to all farmers involved in the study. This research was supported by grants from Iceland, Liechtenstein and Norway (EHP-CZ02-OV-1-027-2015) and by the Charles University, project GA UK (927516).







