If choosing a rechargeable battery for your project intimidates you, [Afroman] has prepared a primer video that should put you at ease. In this tutorial for battery basics he not only walks you through a choice of 5 rechargeable chemistries and their respective tradeoffs, but gives a procedure that will allow you to navigate through the specs of real-world batteries for sale – something that can be the most intimidating part of the process.
You cannot learn everything about batteries in 9 minutes, but watching this should get you from zero to the important 80% of the way there. Even if your project does not give you the specs you need to begin buying, [Afroman] tells you what to measure and how to shop for it. In particular, the information he gives is framed in the context you care about, hopefully ensuring you are not waylaid by all the details that were safe to ignore. If this is not enough, [Afroman]’s prequel video on battery terminology has more detail.
Much like your high school English teacher told you, you need to know the rules before you can choose to break them. Many of battery absolute Dos or Don’ts are written for the manufacturer, who provides for the consumer, not the hacker. Hackaday has published hundreds of battery articles over the years; search our archives when you are ready for more.













afroman is a hero!
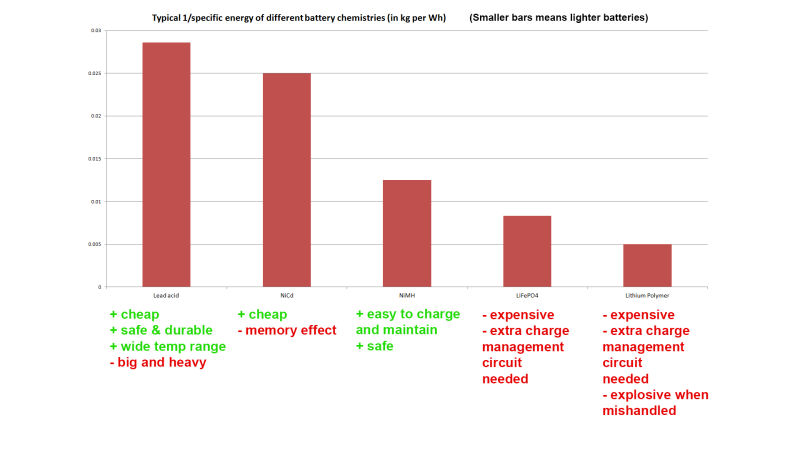
Thanks for the coverage! Btw I made some mistakes in the video – check the annotations and here is an updated comparison chart: https://i.imgur.com/4SJNKPN.png
Power sources, converters, fuels etc are normally compared in terms of energy density. Why did you use 1/energy density?
Because I try to simplify things as much as possible for my audience. By doing 1/ the idea was that people could get a quick visual indicator of the relative weight of different batteries for a given capacity.
I updated our header image to match the corrected one. “expensive” no longer an advantage :p
So, what is a Li-ion? Like the 18650? They say discharge and overcharge protected on the cell. (Though I don’t trust the neat little 16340’s I have that say “Do notineert batteries with the (+) and (-) rewarwed”).
Keep in mind the source video is a 4 minute chemistry and 4 minute purchasing tutorial. If you want something thorough, readers can read for days on this stuff.
*SOME* Lithium Ions have overcharge protection built right into the cell. That is, unless you’re chopping apart the cell, it will prevent you from going below 3.0v or above 4.2v. These are typically ones you would buy on their own and add to a project. It’s certainly not the norm though, many cells are just chemical packages no electronics added.
Lithium Ion and Lithium Polymer are the same chemistry, different way of arranging the chemistry, and almost always LiPos are pouches, not canisters.
Good to know. I bought some one, two and, four cell battery holders for 18650’s and now it sounds like I will not need as much circuitry to charge them, at least the single cell. Also explains the very low cost of the chargers.
I recommend the Efest LUC V4 for an external charger… It’s been reliable for me for almost a year now without any problems.
The protection circuits is only a safety device and not a charger replacement. Even then, the safety records are not that good. Not a good idea to go without a proper charger.
A proper charger chip is about $1 a piece and you can buy charger boards from China not much more. Use a charge chip if you don’t want your battery to have an early retirement due to over charging.
The nice thing is that the unprotected 26650 and even some unprotected 18650’s such as Sony V4 & V5’s are rated for fairly high safe discharge currents… which is excellent for certain short-duty cycle high-draw projects without burning through power sources.
I’m waiting for the 32650’s to become more commonly available from the quality manufacturers(Sony, Panasonic, etc.)
Stating that NiCd chemistry present pemory effect is an overstatment. Also NiMH could present memory effects under the same conditions then NiCd. Memory effects is not the cause of deffect when any of these three criteria is met :
– Batteries achieve full overcharge.
– Discharge is not exactly the same each cycle, within plus or minus 3%
– Discharge is to less than 1.0 volt per cell
Also :
The original paper describing the memory effect was written by GE scientists at their Battery Business Department in Gainesville, Florida, and later retracted by them, but the damage was done. It is unlikely to be a real phenomenon, but has taken on a life of its own as an urban myth.
Proper battery maintenance prevent any memory effect and normal terestrial uses induce variable discharges. Memory effect was only practically observed with satellites on constant 24 hours period…
http://en.wikipedia.org/wiki/Memory_effect
http://en.wikipedia.org/wiki/Nickel%E2%80%93cadmium_battery#Memory_effect
Since Cd in NiCd (Cadmium) is on the RoHS ban list, most of the battery manufacturers have moved to NiMH batteries. A few years ago when I checked (for industrial use at work), it was down to 2 good battery brands making them other than ones from China.
What about us deaf guys who can’t derive much from YouTube video? Got that in text?
My videos have subtitles…
WHAT IF I CAN’T READ THOUGH?
Here’s a more complete overview of lithium chemistries.
http://batteryuniversity.com/learn/article/lithium_based_batteries
Most of my projects work with 2-3.6v, so I use a couple nimh or a single cr2032. Cheap and widely available in local stores.
One of the nice things about the LiFePO4 chemistry is that you can buy drop-in replacements of the same size, but usually greater amp-hours, to replace many standard size SLA batteries. They have the protection circuitry built-in, and are also lighter weight. All that does come with a price – they typically cost 3-4x what the same SLA costs. But if you need the advantages of the lighter weight, it might be worth it.
From what I remember NiMH batteries are actually harder to charge correctly than lithium ion/polymer. Lithium ion charging’s a fairly simple constant-current/constant-voltage affair, whereas NIMH batteries need tricky charge termination circuitry if you want to charge them in a reasonable amount of time without them going pop. See for example http://www.powerstream.com/NiMH.htm
For most people, a constant current charge for NiMH will suffice. They may not get the highest capacity possible out of their cell, but as long as they don’t over-charge it, they should be fine. But to charge a Li-ion cell above 70% capacity or so, you have the constant “high” current portion of the charging cycle first, then when the cell gets to 4.2V (or whatever the max voltage for that given charger is) it switches to a trickle-charge mode for the rest of the until it is fully charged.
This video ignored battery life, self-discharge other than NiMh, and many other factors. LiFePO4 when charged and discharged at less than C/2 are much more robust than implied. (You can kill them with overcharging or overdischarging, but that is true of any chemestry.) I’d love to be able to get LiFePO4 for my laptop so I don’t have to replace it every 10 months.
“This video ignored battery life, [,,,] and many other factors.”
Correct. The chemistry portion of this video was 4 minutes long.
LiFePO4 have lower voltages and lower capacity (i.e. less W*Hr in a given size) than the equivalent Li-ion battery currently in your laptop, so less operating hours per charge. BTW keeping the laptop battery charged at 100% is a good way of killing it.
One very nice thing about them is that their voltage is in the right range of running modern 3.3V chips without requiring a LDO. Still a good idea of using one with low operating current just to have short circuit protection.
Greetings, teekieneet! I’d like to discuss with you the possibilities of hiring you for an engineering project and have been trying to get in contact with you since a few days. I’ve left a message with our email address on the About page of your blog (given that you are the tekkieneet in Ottawa). Looking forward to hear from you! Best, André
I’ll email you. Thanks.
Got it and already replied to it. If it’s not in your Inbox, make sure to check your spam folder.
Please tell me the web site you were using to select a battery- it looked veryuseful, but you only mentioned the name briefly.
I’ve used it before, it’s batteryspace.com
Something is seriously ignored when it comes to LiFePO4…..
NiMH – is a joke ( for “some” (lobby reason !? still domination in Europe )
This “guide” seems to indicate NiMH is the way to go “easy to charge and maintain safe” I have tried in depth working with the best brands of chargers and batteries for NiMh. I would prefer using the following description low voltage ( you HAVE to put them in serial for flashlight GPS ). Less then 500 cycles, expensive…low energy (AA is a joke ). CHARGE MANAGEMENT CIRCUIT DEFINITELY NEEDED
Since I’ve tried LiFePO4 there is no way in hell I’ll buy a NiMH ( please spare me ). In the future I have big hopes for graphene batteries
Charge termination for NiMH is very hard to implement. It is hard to detect changes in tens of mV when you are trying to charge at a fraction of C (some say 1/2C). Just a bit of bad contact in a battery holder / contact resistance changes due to vibration / switcher ripples etc would have messed up the peak detection. Once you have missed that, you have to fall back on secondary means (temperature, timer)
For Li * chemistry, you can easily detect the charge current dropping for termination very easily.
AA NiMH is about 2500mAHr these days (give or take) where as the LiFePO4 I got from DX is about 650mAHr.
650mA*Hr * 3.3V = 2.145 W *Hr vs vs 2500mA * Hr * 1.2V = 3W * Hr. NiMH wins out on energy density. You can probably shop around and get slightly better LiFe, but so can I for NiMH.
NiMH wins is when you can use it in electronics that are designed with AA in mind. For LiFePO4, its voltage is twice as high. You’ll either need a dummy (shorted) battery in those equipment or have to mod the equipment. So effectively, your energy density for 2 AA equivalent is halved when you use a LiFePO4 + a dummy battery.
Now you can use the LiFePO4 capacity more fully than the NiMH which are borderline to low battery for some electronics. I love them for my aging camera.