Key Points
-
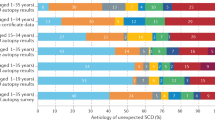
Brugada syndrome is an inherited disease responsible for a large number of sudden deaths in young people without structural heart anomalies
-
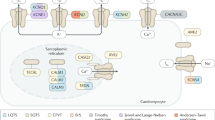
The pathophysiology of Brugada syndrome is unclear; repolarization–depolarization abnormalities underlying this disease can present with different features in different patients
-
A common electrocardiogram (ECG) phenotype seen in patients with Brugada syndrome might be the result of different pathophysiological mechanisms
-
Symptom-type, a spontaneous type 1 ECG pattern, the presence of sinus node dysfunction, and inducible ventricular arrhythmias during programmed stimulation of the heart might help to stratify patients by risk
-
Implantable cardioverter–defibrillator therapy is the most accepted treatment strategy for at-risk patients
-
Electrophysiological-guided therapy with quinidine and radiofrequency substrate ablation might also have a role in the management of these patients
Abstract
Brugada syndrome is an inherited disease characterized by an increased risk of sudden cardiac death owing to ventricular arrhythmias in the absence of structural heart disease. Since the first description of the syndrome >20 years ago, considerable advances have been made in our understanding of the underlying mechanisms involved and the strategies to stratify at-risk patients. The development of repolarization–depolarization abnormalities in patients with Brugada syndrome can involve genetic alterations, abnormal neural crest cell migration, improper gap junctional communication, or connexome abnormalities. A common phenotype observed on the electrocardiogram of patients with Brugada syndrome might be the result of different pathophysiological mechanisms. Furthermore, risk stratification of this patient cohort is critical, and although some risk factors for Brugada syndrome have been frequently reported, several others remain unconfirmed. Current clinical guidelines offer recommendations for patients at high risk of developing sudden cardiac death, but the management of those at low risk has not yet been defined. In this Review, we discuss the proposed mechanisms that underlie the development of Brugada syndrome and the current risk stratification and therapeutic options available for these patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brugada, P. & Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 20, 1391–1396 (1992).
Antzelevitch, C. et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 111, 659–670 (2005).
Nademanee, K. et al. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation 96, 2595–2600 (1997).
Tan, H. L., Hofman, N., van Langen, I. M., van der Wal, A. C. & Wilde, A. A. Sudden unexplained death: heritability and diagnostic yield of cardiological and genetic examination in surviving relatives. Circulation 112, 207–213 (2005).
van der Werf, C. et al. Diagnostic yield in sudden unexplained death and aborted cardiac arrest in the young: the experience of a tertiary referral center in The Netherlands. Heart Rhythm 7, 1383–1389 (2010).
Mellor, G. et al. Clinical characteristics and circumstances of death in the sudden arrhythmic death syndrome. Circ. Arrhythm. Electrophysiol. 7, 1078–1083 (2014).
Garson, A. Jr et al. The long QT syndrome in children. An international study of 287 patients. Circulation 87, 1866–1872 (1993).
Kapplinger, J. D. et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 7, 33–46 (2010).
Hu, D. et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J. Am. Coll. Cardiol. 64, 66–79 (2014).
Nielsen, M. W., Holst, A. G., Olesen, S. P. & Olesen, M. S. The genetic component of Brugada syndrome. Frontiers Physiol. 4, 179 (2013).
Dendramis, G., Antzelevitch, C. & Brugada, P. Brugada Syndrome: Diagnosis, Clinical Manifestations, Risk Stratification and Treatment. (Nova Science Publishers, 2015).
Sarquella-Brugada, G., Campuzano, O., Arbelo, E., Brugada, J. & Brugada, R. Brugada syndrome: clinical and genetic findings. Genet. Med. 18, 3–12 (2016).
Ueda, K. et al. Role of HCN4 channel in preventing ventricular arrhythmia. J. Hum. Genet. 54, 115–121 (2009).
Liu, H. et al. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS ONE 8, e54131 (2013).
Syam, N. et al. Variants of transient receptor potential melastatin member 4 in childhood atrioventricular block. J. Am. Heart Assoc. 5, e001625 (2016).
Sato, P. Y. et al. Interactions between ankyrin-G, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ. Res. 109, 193–201 (2011).
Sato, P. Y. et al. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ. Res. 105, 523–526 (2009).
Cerrone, M. et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 129, 1092–1103 (2014).
Bezzina, C. R. et al. Common variants at SCN5A- SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 45, 1044–1049 (2013).
Campuzano, O., Brugada, R. & Iglesias, A. Genetics of Brugada syndrome. Curr. Opin. Cardiol. 25, 210–215 (2010).
Crotti, L. et al. Spectrum and prevalence of mutations involving BrS1- through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J. Am. Coll. Cardiol. 60, 1410–1418 (2012).
Le Scouarnec, S. et al. Testing the burden of rare variation in arrhythmia-susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Hum. Mol. Genet. 24, 2757–2763 (2015).
Ackerman, M. J. et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8, 1308–1339 (2011).
Yan, G. X. & Antzelevitch, C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 100, 1660–1666 (1999).
Bloch Thomsen, P. E. et al. Phase 2 reentry in man. Heart Rhythm 2, 797–803 (2005).
Antzelevitch, C. In vivo human demonstration of phase 2 reentry. Heart Rhythm 2, 804–806 (2005).
Szel, T. & Antzelevitch, C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of Brugada syndrome. J. Am. Coll. Cardiol. 63, 2037–2045 (2014).
Koncz, I. et al. Mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of early repolarization syndrome. J. Mol. Cell. Cardiol. 68, 20–28 (2014).
Patocskai, B. & Antzelevitch, C. Novel therapeutic strategies for the management of ventricular arrhythmias associated with the Brugada syndrome. Expert Opin. Orphan Drugs 3, 633–651 (2015).
Coronel, R. et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation 112, 2769–2777 (2005).
Nademanee, K. et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 123, 1270–1279 (2011).
Boukens, B. J. et al. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ. Res. 113, 137–141 (2013).
van Rijen, H. V. et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109, 1048–1055 (2004).
Moorman, A. F. & Christoffels, V. M. Cardiac chamber formation: development, genes, and evolution. Physiol. Rev. 83, 1223–1267 (2003).
Borggrefe, M. & Schimpf, R. J-wave syndromes caused by repolarization or depolarization mechanisms a debated issue among experimental and clinical electrophysiologists. J. Am. Coll. Cardiol. 55, 798–800 (2010).
Elizari, M. V. et al. Abnormal expression of cardiac neural crest cells in heart development: a different hypothesis for the etiopathogenesis of Brugada syndrome. Heart Rhythm 4, 359–365 (2007).
Waldo, K. L., Lo, C. W. & Kirby, M. L. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Dev. Biol. 208, 307–323 (1999).
Sohl, G. & Willecke, K. Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232 (2004).
Kumar, N. M. & Gilula, N. B. The gap junction communication channel. Cell 84, 381–388 (1996).
Ewart, J. L. et al. Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development 124, 1281–1292 (1997).
Dhar Malhotra, J. et al. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation 103, 1303–1310 (2001).
Malhotra, J. D., Kazen-Gillespie, K., Hortsch, M. & Isom, L. L. Sodium channel beta subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 275, 11383–11388 (2000).
Jansen, J. A. et al. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 9, 600–607 (2012).
Desplantez, T. et al. Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc. Res. 94, 58–65 (2012).
Lubkemeier, I. et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Bas. Res. Cardiol. 108, 348 (2013).
Poelzing, S., Akar, F. G., Baron, E. & Rosenbaum, D. S. Heterogeneous connexin43 expression produces electrophysiological heterogeneities across ventricular wall. Am. J. Physiol. Heart Circulatory Physiol. 286, H2001–2009 (2004).
Vaidya, D. et al. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ. Res. 88, 1196–1202 (2001).
Nademanee, K. et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J. Am. Coll. Cardiol. 66, 1976–1986 (2015).
Agullo-Pascual, E., Cerrone, M. & Delmar, M. Arrhythmogenic cardiomyopathy and Brugada syndrome: diseases of the connexome. FEBS Lett. 588, 1322–1330 (2014).
Rhett, J. M. & Gourdie, R. G. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9, 619–623 (2012).
Petitprez, S. et al. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ. Res. 108, 294–304 (2011).
Rhett, J. M., Veeraraghavan, R., Poelzing, S. & Gourdie, R. G. The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovascular Med. 23, 222–228 (2013).
Zareba, W. et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N. Engl. J. Med. 339, 960–965 (1998).
Webster, G. & Berul, C. I. Congenital long-QT syndromes: a clinical and genetic update from infancy through adulthood. Trends Cardiovascular Med. 18, 216–224 (2008).
Garcia, J. et al. Clinical genetic testing for the cardiomyopathies and arrhythmias: a systematic framework for establishing clinical validity and addressing genotypic and phenotypic heterogeneity. Front. Cardiovasc.Med. 3, 20 (2016).
Dendramis, G. Coronary anomalies and Brugada phenocopy, the first documented case in the world. Int. J. Cardiol. 199, 335–336 (2015).
Priori, S. G. Natural history of Brugada syndrome: insights for risk stratification and management. Circulation 105, 1342–1347 (2002).
Sieira, J. et al. Prognostic value of programmed electrical stimulation in Brugada syndrome: 20 years experience. Circ. Arrhythm. Electrophysiol. 8, 777–784 (2015).
Probst, V. et al. Long-term prognosis of patients diagnosed with Brugada syndrome: results from the FINGER Brugada Syndrome Registry. Circulation 121, 635–643 (2010).
Priori, S. G. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 36, 2793–2867 (2015).
Priori, S. G. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963 (2013).
Olde Nordkamp, L. R. et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 13, 443–454 (2016).
Conte, G. et al. Implantable cardioverter-defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J. Am. Coll. Cardiol. 65, 879–888 (2015).
Sacher, F. et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation 128, 1739–1747 (2013).
Brugada, J. et al. Brugada syndrome phenotype elimination by epicardial substrate ablation. Circ. Arrhythm. Electrophysiol. 8, 1373–1381 (2015).
Belhassen, B., Rahkovich, M., Michowitz, Y., Glick, A. & Viskin, S. Management of Brugada syndrome: thirty-three-year experience using electrophysiologically guided therapy with class 1A antiarrhythmic drugs. Circ. Arrhythm. Electrophysiol. 8, 1393–1402 (2015).
Sroubek, J. et al. Programmed ventricular stimulation for risk stratification in the Brugada syndrome: a pooled analysis. Circulation 133, 622–630 (2016).
Sieira, J. et al. Clinical characterisation and long-term prognosis of women with Brugada syndrome. Heart 102, 452–458 (2016).
Bagnall, R. D. et al. Prospective study of sudden cardiac death among children and young adults. N. Engl. J. Med. 374, 2441–2452 (2016).
Priori, S. G. et al. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 59, 37–45 (2012).
Delise, P. et al. Risk stratification in individuals with the Brugada type 1 ECG pattern without previous cardiac arrest: usefulness of a combined clinical and electrophysiologic approach. Eur. Heart J. 32, 169–176 (2011).
Sieira, J. et al. Asymptomatic Brugada syndrome: clinical characterization and long-term prognosis. Circ. Arrhythm. Electrophysiol. 8, 1144–1150 (2015).
Brugada, J. Long-term follow-up of individuals with the electrocardiographic pattern of right bundle-branch block and ST-segment elevation in precordial leads V1 to V3. Circulation 105, 73–78 (2002).
Deo, R. & Albert, C. M. Epidemiology and genetics of sudden cardiac death. Circulation 125, 620–637 (2012).
Delise, P., Allocca, G., Sitta, N. & DiStefano, P. Event rates and risk factors in patients with Brugada syndrome and no prior cardiac arrest: a cumulative analysis of the largest available studies distinguishing ICD-recorded fast ventricular arrhythmias and sudden death. Heart Rhythm 11, 252–258 (2014).
Andorin, A. et al. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 13, 1274–1282 (2016).
Gonzalez-Corcia, M. et al. Brugada syndrome in the young: assessment of risk factors for future events. Europace (2016).
Conte, G. et al. Follow-up from childhood to adulthood of individuals with family history of Brugada syndrome and normal electrocardiograms. JAMA 312, 2039–2041 (2014).
Conte, G. et al. Clinical characteristics, management, and prognosis of elderly patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 25, 514–519 (2014).
Benito, B. et al. Gender differences in clinical manifestations of Brugada syndrome. J. Am. Coll. Cardiol. 52, 1567–1573 (2008).
Sacher, F. et al. Are women with severely symptomatic Brugada syndrome different from men? J. Cardiovasc. Electrophysiol. 19, 1181–1185 (2008).
Sarkozy, A. et al. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur. Heart J. 32, 2153–2160 (2011).
Hasdemir, C. et al. High prevalence of concealed Brugada syndrome in patients with atrioventricular nodal reentrant tachycardia. Heart Rhythm 12, 1584–1594 (2015).
Sommariva, E. et al. Genetics can contribute to the prognosis of Brugada syndrome: a pilot model for risk stratification. Eur. J. Hum. Genet. 21, 911–917 (2013).
Meregalli, P. G. et al. Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm 6, 341–348 (2009).
Richter, S. et al. Number of electrocardiogram leads displaying the diagnostic coved-type pattern in Brugada syndrome: a diagnostic consensus criterion to be revised. Eur. Heart J. 31, 1357–1364 (2010).
Miyamoto, K. et al. Diagnostic and prognostic value of a type 1 Brugada electrocardiogram at higher (third or second) V1 to V2 recording in men with Brugada syndrome. Am. J. Cardiol. 99, 53–57 (2007).
Gottschalk, B. H. et al. Expert cardiologists cannot distinguish between Brugada phenocopy and Brugada syndrome electrocardiogram patterns. Europace 18, 1095–1100 (2016).
Calo, L. et al. Am. Coll. Cardiol. 67, 1427–1440 (2016).
Kawata, H. et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: a novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm 10, 1161–1168 (2013).
Takagi, M. et al. The prognostic value of early repolarization (J wave) and ST-segment morphology after J wave in Brugada syndrome: multicenter study in Japan. Heart Rhythm 10, 533–539 (2013).
Alings M, Wilde A. “Brugada” syndrome: clinical data and suggested pathophysiological mechanism. Circulation 99, 666–673 (1999).
Miyazaki, T. et al. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J. Am. Coll. Cardiol. 27, 1061–1070 (1996).
Mizumaki, K. et al. Vagal activity modulates spontaneous augmentation of ST elevation in the daily life of patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 15, 667–673 (2004).
Viskin, S., Rosso, R., Friedensohn, L., Havakuk, O. & Wilde, A. A. Everybody has Brugada syndrome until proven otherwise? Heart Rhythm 12, 1595–1598 (2015).
Havakuk, O. & Viskin, S. A. A tale of 2 diseases: the history of long-QT syndrome and Brugada syndrome. J. Am. College Cardiol. 67, 100–108 (2016).
Brugada, R. et al. Sodium channel blockers identify risk for sudden death in patients with ST-segment elevation and right bundle branch block but structurally normal hearts. Circulation 101, 510–515 (2000).
Morita, H. et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 118, 1697–1704 (2008).
Okamura, H. et al. Risk stratification in patients with Brugada syndrome without previous cardiac arrest - prognostic value of combined risk factors. Circ. J. 79, 310–317 (2015).
Sarkozy, A. et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2, 154–161 (2009).
Kamakura, T. et al. Significance of electrocardiogram recording in high intercostal spaces in patients with early repolarization syndrome. Eur. Heart J. 37, 630–637 (2016).
Zumhagen, S. et al. Tpeak-Tend interval and Tpeak-Tend/QT ratio in patients with Brugada syndrome. Europace http://dx.doi.org/10.1093/europace/euw033 (2016).
Castro Hevia, J. et al. Tpeak-Tend and Tpeak-Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J. Am. Coll. Cardiol. 47, 1828–1834 (2006).
Babai Bigi, M. A., Aslani, A. & Shahrzad, S. aVR sign as a risk factor for life-threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm 4, 1009–1012 (2007).
Uchimura-Makita, Y. et al. Time-domain T-wave alternans is strongly associated with a history of ventricular fibrillation in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 25, 1021–1027 (2014).
Morita, H. et al. Atrial fibrillation and atrial vulnerability in patients with Brugada syndrome. J. Am. Coll. Cardiol. 40, 1437–1444 (2002).
Rodriguez-Manero, M. et al. Prevalence, clinical characteristics and management of atrial fibrillation in patients with Brugada syndrome. Am. J. Cardiol. 111, 362–367 (2013).
Giustetto, C. et al. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm 11, 259–265 (2014).
Morita, H. et al. Sinus node function in patients with Brugada-type ECG. Circ. J. 68, 473–476 (2004).
Letsas, K. P. et al. Sinus node disease in subjects with type 1 ECG pattern of Brugada syndrome. J. Cardiol. 61, 227–231 (2013).
Abe, K. et al. Sodium channelopathy underlying familial sick sinus syndrome with early onset and predominantly male characteristics. Circ. Arrhythm. Electrophysiol. 7, 511–517 (2014).
Brugada, P., Geelen, P., Brugada, R., Mont, L. & Brugada, J. Prognostic value of electrophysiologic investigations in Brugada syndrome. J. Cardiovasc. Electrophysiol. 12, 1004–1007 (2001).
Eckardt, L. et al. Electrophysiologic investigation in Brugada syndrome; yield of programmed ventricular stimulation at two ventricular sites with up to three premature beats. Eur. Heart J. 23, 1394–1401 (2002).
Takigawa, M. et al. Seasonal and circadian distributions of ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm 5, 1523–1527 (2008).
Stroker, E., de Asmundis, C., Chierchia, G. B. & Brugada, P. Exercise-related Brugada pattern and monomorphic ventricular tachycardia in a patient with Brugada syndrome: interplay between body temperature, haemodynamics and vagal activity. Eur. Heart J. 37, 655 (2016).
Belhassen, B., Glick, A. & Viskin, S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 110, 1731–1737 (2004).
Anguera, I. et al. Shock reduction with long-term quinidine in patients with Brugada syndrome and malignant ventricular arrhythmia episodes. J. Am. Coll. Cardiol. 67, 1653–1654 (2016).
Eckardt, L. et al. Long-term prognosis of individuals with right precordial ST-segment-elevation Brugada syndrome. Circulation 111, 257–263 (2005).
Takagi, M., Yokoyama, Y., Aonuma, K., Aihara, N. & Hiraoka, M. Clinical characteristics and risk stratification in symptomatic and asymptomatic patients with brugada syndrome: multicenter study in Japan. J. Cardiovasc. Electrophysiol. 18, 1244–1251 (2007).
Brugada, J., Brugada, R. & Brugada, P. Determinants of sudden cardiac death in individuals with the electrocardiographic pattern of Brugada syndrome and no previous cardiac arrest. Circulation 108, 3092–3096 (2003).
Author information
Authors and Affiliations
Contributions
J.S. and G.D. contributed equally to this work. All the authors researched data for the article, substantially contributed to discussion of content, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.B. has received institutional grants from Biotronik, Medtronic, and St. Jude Medical. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Sieira, J., Dendramis, G. & Brugada, P. Pathogenesis and management of Brugada syndrome. Nat Rev Cardiol 13, 744–756 (2016). https://doi.org/10.1038/nrcardio.2016.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.143
This article is cited by
-
Anesthesia in patients with Brugada syndrome: two case reports
Journal of Medical Case Reports (2023)
-
Epicardial Dispersion of Repolarization Promotes the Onset of Reentry in Brugada Syndrome: A Numerical Simulation Study
Bulletin of Mathematical Biology (2023)
-
Novel SCN5A variants identified in a group of Iranian Brugada syndrome patients
Functional & Integrative Genomics (2021)
-
Development and diversity of a novel panel of short tandem repeat markers encompassing the SCN5A gene in Iranian population
Journal of Genetics (2018)
-
Unsafe Drug Use and Arrhythmic Events in Brugada Patients with ICD: Results of a Long-Term Follow-Up
Cardiovascular Drugs and Therapy (2018)