Advances Across Urothelial Carcinomas Highlight Need for Shared Decision-making
As longer-term data read out for established regimens in urothelial cancer and up-and-coming therapeutics inch toward approval, optimizing treatment approaches relies on buy-in from multidisciplinary teams and patients.
Petros Grivas, MD, PhD

As longer-term data read out for established regimens in urothelial cancer and up-and-coming therapeutics inch toward approval, optimizing treatment approaches relies on buy-in from multidisciplinary teams and patients.
“It’s great to see how the field is rapidly evolving and how we can provide more options for our patients,” Petros Grivas, MD, PhD, said in a recent OncLive Peer Exchange. “In the past 6 years, we have all these new agents— checkpoint inhibitors, 2 antibody-drug conjugates, targeted therapy— all recently approved and many more to come.”
As more options expand the use of systemic therapies in practice to either avoid or reduce the invasiveness of surgical approaches, discussions across teams as well as with patients are vital. “It’s a balanced discussion about the risks and benefits of trimodal therapy vs surgery,” Mamta Parikh, MD, MS, said. “We often have patients who see radiation oncologists and surgeons….It is important for them to get both perspectives. It comes down to the individual patient—what they’re willing to undergo—and I think clinical trials are so important in this area because I do feel that we may be on the cusp of getting to real bladder preservation for all of our patients with muscle-invasive disease.”
Parikh and Grivas joined a panel of genitourinary cancer experts to unpack how the latest data presented at the 2023 American Society of Clinical Oncology Genitourinary Cancers (ASCO GU) Symposium will influence practice for patients with urothelial carcinomas.
Following Avenues of Efficacy
Most patients with high-risk, non–muscle-invasive bladder cancer (NMIBC) with cancer that does not respond or recurs within 12 months have a poor prognosis and will require a radical cystectomy.1
The single-arm, multicohort phase 2 KEYNOTE-057 trial (NCT02625961) evaluated the safety and efficacy of the PD-1 inhibitor pembrolizumab (Keytruda) monotherapy in patients with BCG-unresponsive high-risk NMIBC who were ineligible or declined to undergo radical cystectomy. Results from cohort A supported the FDA approval of pembrolizumab monotherapy for this patient population in January 2020.2
Cohort B of KEYNOTE-057 included patients with papillary tumors without carcinoma in situ (CIS) and the primary end points were 12-month disease-free survival (DFS) rate of high-risk NMIBC and complete response (CR) rate. After approximately 45 months of follow-up, pembrolizumab showed notable antitumor activity in patients with non-CIS papillary high-risk NMIBC unresponsive to BCG. There were no new safety concerns, and the toxicity was manageable and consistent with cohort A. Results indicate that pembrolizumab monotherapy may be beneficial for patients with non-CIS papillary high-risk NMIBC that is refractory to BCG and who also declined or were ineligible for radical cystectomy.1
Among patients in this cohort (n = 132), the 12-month DFS rate for high-risk NMIBC rate was 43.5% (95% CI, 34.9%-51.9%). The 24- and 36-month DFS rates were both estimated to be 34.9% (95% CI, 26.4%-43.4%). The median DFS was 7.7 months (95% CI, 5.5-13.6). Regarding DFS for any disease, the 12-month DFS rate was 41.7% (95% CI, 33.1%-50.0%), and the 24-, and 36-month DFS rates were both 33.0% (95% CI, 24.7%-41.5%). Of note, trial investigators called attention to patients with progressive disease at baseline (n = 18), for whom the 12-month DFS rate was 23.5% (95% CI, 7.3%-44.9%).1
Progression-free survival (PFS) outcomes were also favorable for pembrolizumab in cohort B, with a 12-month PFS rate of 88.2% (95% CI, 80.0%-93.2%) for worsening of grade, stage, or death and 88.2% (95% CI, 79.4%-93.3%) for progression to metastatic disease or death. The median PFS for grade/stage was 44.5 months (95% CI, 34.6-not estimable [NE]) and 46.2 months (95% CI, 36.8-NE) for invasive or metastatic disease.1
“There was very good disease control, a very high cystectomy-free rate,” Grivas noted adding that “the toxicity signal was what we would expect and there were no new safety signals. Something to look at in the non–muscle-invasive disease space.”
To build on these signals, novel combinations are being explored to boost efficacy with pembrolizumab. For example, the immune checkpoints TIGIT and LAG-3 are known to contribute to treatment resistance in many cancers, so investigators are seeking to leverage these with dual-inhibition approaches. Thus, cohort C of the KEYNOTE-057 trial will evaluate the efficacy and safety of pembrolizumab and the TIGIT inhibitor vibostolimab or the LAG-3 inhibitor favezelimab in patients with high-risk BCG-unresponsive NMIBC with CIS with or without papillary tumors. Tumor evaluations will take place every 12 weeks until year 2 and then every 24 weeks for up to 5 years. The primary efficacy end point is the 12-month complete response rate of high-risk NMIBC.3
“There’s a lot of efforts right now in terms of intravesical therapy and trying to develop larger trials for BCG-unresponsive disease and studies that also include randomization,” Andrea B. Apolo, MD, said. “There’s a lot of activity right now for patients with NMIBC that are BCG unresponsive.”
Leveraging Findings From Adjuvant Therapy Trials
Patients with muscle-invasive urothelial carcinoma (MIUC) have a high recurrence rate despite treatment with neoadjuvant cisplatin-based chemotherapy, followed by radical cystectomy in eligible patients.4 Immunotherapy for patients with high-risk muscle-invasive disease has been established in the adjuvant setting with agents such as nivolumab (Opdivo) demonstrating durable improvements.4,5 Other treatment options, such as atezolizumab (Tecentriq), have missed the mark in this population, and the role of pembrolizumab in this setting remains to be seen.6
The approval of nivolumab followed the DFS primary end point in the phase 3 CheckMate 274 trial (NCT02632409) being met in the intention-to-treat (ITT) group and in patients with PD-L1 expression of at least 1%.4,5 Specifically, CheckMate 274 enrolled patients with highrisk MIUC after radical resection. Patients were randomly assigned 1:1 to nivolumab (n = 353) or placebo (n = 356) for a maximum of 1 year of treatment. Patients had pathologic evidence of UC at high risk of recurrence and an ECOG performance status 1 or less. Primary end points were DFS in ITT patients and in patients with PD-L1 expression of at least 1%. DFS was also analyzed in prespecified subgroups.4
At a median follow-up of 36.1 months, the median DFS was 22.0 months (95% CI, 18.8-36.9) with nivolumab arm vs 10.9 months (95% CI, 8.3-15.2) with placebo in ITT patients. Additionally, the median DFS was 52.6 months (95% CI, 25.8-NE) with nivolumab (n = 140) vs 8.4 months (95% CI, 5.6-17.9) with placebo (n = 142) in patients with PD-L1 expression of at least 1%. DFS benefit was seen in most subgroups analyzed. Safety data were consistent with the primary analysis. Overall survival (OS) will be evaluated. At follow-up, nivolumab continued to show DFS benefits vs placebo. These results further support adjuvant nivolumab as a standard of care for patients with high-risk MIUC after radical resection.4
“Adjuvant chemotherapy has been used for years and has shown an improvement in PFS,” Apolo said. “There are important data showing that chemotherapy immediately after surgery does improve outcomes, although the trial did not fully accrue to give the OS benefit. We do see a benefit when patients are treated earlier with systemic therapy. If the patient already received neoadjuvant therapy, then we can talk about immunotherapy.”
The phase 3 IMvigor010 trial (NCT02450331) compared atezolizumab with observation as adjuvant therapy in patients with high-risk MIUC. IMvigor010 is, to our knowledge, the largest, first-completed phase 3 adjuvant study to evaluate the role of a checkpoint inhibitor in MIUC, Shilpa Gupta, MD, explained. The trial did not meet its primary end point of improved DFS with adjuvant atezolizumab, and the use of adjuvant checkpoint inhibitor therapy in the setting evaluated in IMvigor010 is not supported at this time.6
“It’s important to enroll on trials…to address some of these unmet needs,” Gupta said. “We learned a lot from IMvigor010. Although it was a negative trial for the primary end point, it did teach us a lot about minimal residual disease [MRD] in these patients.”
Gupta noted a poster presentation on circulating tumor DNA (ctDNA) analysis in the IMvigor010 study given during the 2023 ASCO GU Symposium.7 Investigators reported that ctDNA detection at a postsurgical time point was prognostic for DFS and OS. In the observation arm of the trial, patients were stratified by positive (n = 66) or negative (n = 118) ctDNA status. The median DFS was 3 months for those with ctDNA positivity vs not reached for those with ctDNA negativity (HR, 4.86; 95% CI, 3.09-7.60; P < .0001). The median OS was 13 months vs not reached, respectively (HR, 5.47; 95% CI, 3.19-9.40; P < .0001).7
“We’re working in exciting times right now where we have some biomarkers that may be able to select the patients that respond better or benefit the most,” Apolo said. “The problem with adjuvant therapy is that we overtreat patients. That’s why it’s hard to see the benefit that the therapy does because a lot of the patients may be cured, and that’s true of all forms. It’s hard to select the patients [who] meet the [criteria for] therapy. That’s why I think ctDNA is very exciting.”
“I have not started using the FDA-approved ctDNA test in clinical practice,” Parihk said. “We have some trials that are evaluating this, but as of now I think that it’s a fascinating area and the IMvigor data are interesting, but I’m not ready to allow it to guide my clinical practice quite yet. We need more trials that will help us further determine how to incorporate MRD into our practice.”
Other trials examining adjuvant immunotherapy include the phase 3 AMBASSADOR trial (NCT03244384), which is exploring the use of pembrolizumab in patients with localized MIBC and locally advanced UC. Monoclonal antibodies can enhance the immune response and help fight cancer by recognizing and blocking checkpoint molecules. OS and DFS are the primary outcome measures in this study, which is expected to complete in 2025.8 “I’m awaiting the AMBASSADOR trial results and would be very excited to report that out,” Apolo said. “It’s such an important trial because the CheckMate-274 [trial] is showing an improvement in DFS. We really want to see what other agents show since the IMvigor010 did not show the data. I’m excited about the AMBASSADOR trial.”
In the clinic, Apolo said that she discusses adjuvant therapy options based on established findings and takes the patient’s prior courses of therapy into consideration. “I do discuss adjuvant chemotherapy with patients if they have not received chemotherapy,” she said. “If they’ve already received chemotherapy, then I discuss adjuvant checkpoint inhibitor [therapy] with nivolumab and discuss the PFS data. We still don’t have survival data, but the PFS data are positive, and it does look like it improves outcomes in patients in terms of DFS in the adjuvant setting, so it is something that I will discuss with patients and offer it to them.
“In terms of upper tract [disease] that’s a little harder. We don’t want to base decisions on subgroup analyses, and that’s what we have right now for adjuvant checkpoint [inhibitors]. The subgroup analysis in patients with upper tract disease did not look like it benefitted them as much as the bladder primaries. If a patient has already received chemotherapy, then I also discuss adjuvant nivolumab; if they haven’t, then I discuss adjuvant chemotherapy.”
Zeroing in on Targeted Therapy
On April 3, 2023, the FDA granted accelerated approval to enfortumab vedotin-ejfv (Padcev) in combination with pembrolizumab for patients with locally advanced or metastatic UC and who are ineligible for cisplatin chemotherapy. The second indication for the antibody-drug conjugate highlights the role of nectin-4 as a clinically actionable target in this patient population.9
The most recent approval was supported by findings from the phase 1/2 KEYNOTE-869 trial (NCT03288545), which showed that the objective response rate in patients that received enfortumab vedotin and pembrolizumab (n = 121) was 68% (95% CI, 59%-76%), including a 12% CR rate.9 The recommended enfortumab vedotin dose given with pembrolizumab is 1.25 mg/kg (up to 125 mg for patients with a body weight ≥ 100 kg) via intravenous infusion over 30 minutes on days 1 and 8 of a 21-day cycle until disease progression or unacceptable toxicity. The recommended pembrolizumab dose, administered after enfortumab vedotin on the same day, is 200 mg every 3 weeks or 400 mg every 6 weeks.9
“We have data in the metastatic setting that enfortumab vedotin is active,” Apolo said. “It’s active on its own as monotherapy and it’s active in combination with checkpoint inhibitor pembrolizumab. This year we saw several trials in progress that are comparing enfortumab vedotin alone with enfortumab vedotin in combination with pembrolizumab. The KEYNOTE-905/EV-303 study [NCT03924895] is evaluating neoadjuvant pembrolizumab alone compared with pembrolizumab plus enfortumab vedotin. KEYNOTE-B15/EV-304 [NCT04700124] is a randomized phase 3 trial comparing enfortumab vedotin plus pembrolizumab with platinum-based chemotherapy in the neoadjuvant setting for patients with muscle-invasive disease. It’s going to be very interesting to move these therapies forward and to see what the outcomes are in these trials.”
The phase 2 TROPHY U-01 trial (NCT03547973) is a multicohort, open-label, study that led to the accelerated FDA approval of sacituzumab govitecan-hziy (Trodelvy) for patients with locally advanced or metastatic UC who previously received a platinum-containing chemotherapy and either a PD-1 or PD-L1 inhibitor.10 Neutropenia and diarrhea are the most frequent toxicities associated with sacituzumab govitecan, an active medication with a controllable safety profile.11
“The TROPHY U-01 cohort 5 study investigated sacituzumab govitecan plus zimberelimab, which is a PD1 inhibitor, vs zimberelimab alone vs avelumab [Bavencio],” Parikh said. “It will give us interesting data. It will be challenging for patients to be on that kind of intensified therapy. There are more challenges in terms of weekly infusions, potential for cytopenias, and gastrointestinal [adverse] effects, so we’ll have to see if the tolerability is acceptable. It will be interesting to see the duration of therapy for all these regimens, including with intensified approaches.”
Taking the Whole Patient Into Consideration
Parikh noted that bladder-preserving efforts are something that should be given more focus because patients’ quality of life is greatly affected with radical cystectomies (Table).12 “There are a lot of really exciting things that are around the corner for bladder cancer [and] one thing that’s very inspiring in our field is that we’re listening to the patients about what they want,” she said. “We have heard that cystectomies are [procedures] they would like to avoid, as they are impacting their quality of life. It’s wonderful to see the field listening to that and looking in both the non–muscle-invasive space as well as the muscle-invasive space to find patients for whom we can spare their bladders and looking at approaches that potentially could get us to bladder preservation for all.”
Table. Urothelial Cancer Agents Approved by the FDA Since 202012
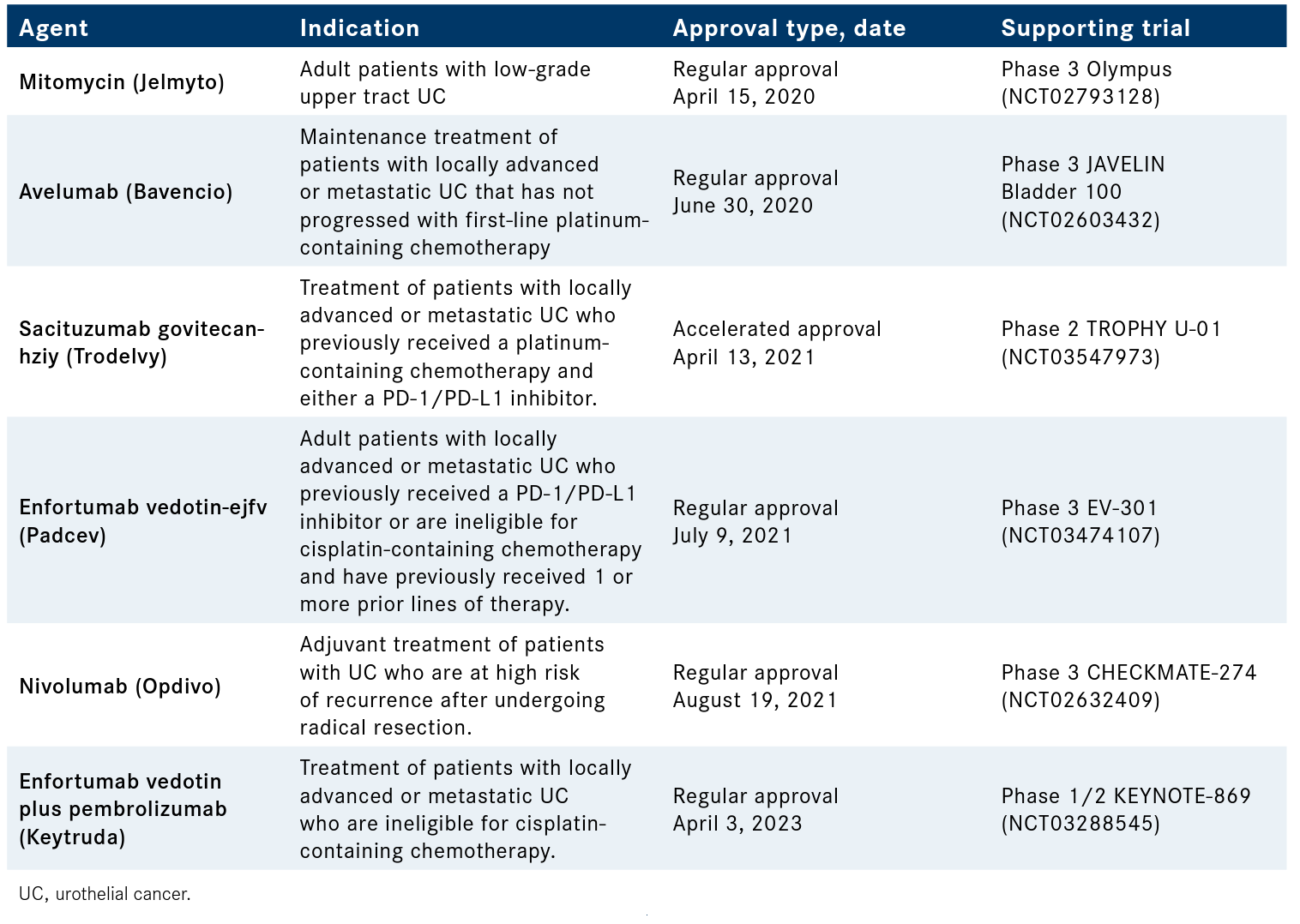
Grivas also noted that novel preservation approaches are actively being discussed in the clinic using the tools that are available. “This is a very important and rapidly evolving theme [as] we see a trend toward a higher desire and willingness to go for bladder preservation,” he said. “This has been evolving with our urologist [colleagues] being strong advocates for that in well-selected patients. The way we navigate discussions in a busy multidisciplinary clinic is we try to think about the individual in front of us. What is the best local regional definitive therapy assuming no metastasis of course and curative intent? Is radical surgery or trimodality therapy the best approach for bladder preservation? This has to do with a number of patient-related characteristics but also cancer-related characteristics.”
Counseling the patient on the best course of action is the next step, and keeping the patient part of the decision-making process is paramount. “With this approach I would say that approximately 15% to 20% of patients may undergo bladder preservation using anecdotal statistics and of course it’s a method of selection based on the criteria we use for bladder preservation,” Grivas said.
References
- Necchi A, Roumiguié M, Esen AA, et al. Pembrolizumab (pembro) monotherapy for patients (pts) with high-risk non-muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette-Guérin (BCG): results from cohort B of the phase 2 KEYNOTE-057 trial. J Clin Oncol. 2023;41(suppl 6):LBA442. doi:10.1200/JCO.2023.41.6_suppl. LBA442
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. FDA. Updated January 8, 2020. Accessed May 10, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer
- Shore ND, Gupta S, Kulkarni GS, et al. Phase 2 KEYNOTE-057 cohort C: pembrolizumab (pembro) with vibostolimab or favezelimab for patients (pts) with high-risk (HR) bacillus Calmette-Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC). J Clin Oncol. 2023;41 (suppl 6):TPS591. doi:10.1200/JCO.2023.41.6_suppl.TPS591
- Galsky M, Witjes AA, Gschwend JE, et al. Extended follow-up results from the CheckMate 274 trial. J Clin Oncol. 2023;41(suppl 6):LBA443. doi:10.1200/JCO.2023.41.6_suppl.LBA443
- FDA approves nivolumab for adjuvant treatment of urothelial carcinoma. FDA. Updated February 1, 2022. Accessed May 11, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma
- Bellmunt J, Hussain M, Gschwend JE, et al; IMvigor010 Study Group. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525-537. doi:10.1016/S14702045(21)00004-8
- Young A, Madison R, Fine AD, et al. Molecular residual disease (MRD) detection with a tissue comprehensive genomic profiling (CGP)-informed personalized monitoring assay: an exploratory analysis of the IMvigor-010 observation arm. J Clin Oncol. 2023;41(suppl 6):448. doi:10.1200/JCO.2022.40.6_suppl.448
- Testing MK-3475 (pembrolizumab) after surgery for localized muscle-invasive bladder cancer and locally advanced urothelial cancer (AMBASSADOR). ClinicalTrials.gov. Updated May 3, 2023. Accessed May 9, 2023. https://clinicaltrials.gov/ct2/show/NCT03244384
- FDA grants accelerated approval to enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial carcinoma. FDA. Updated April 3, 2023. Accessed May 9, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-enfortumab-vedotin-ejfv-pembrolizumab-locally-advanced-or-metastatic
- FDA grants accelerated approval to sacituzumab govitecan for advanced urothelial cancer. FDA. April 13, 2021. Accessed May 10, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sacituzumab-govitecan-advanced-urothelial-cancer
- Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39(22):2474-2485. doi:10.1200/ JCO.20.03489
- Oncology (cancer)/hematologic malignancies approval notifications. FDA. Updated April 20, 2023. Accessed May 12, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/oncology-cancer-hematologic-malignancies-approval-notifications

Latest Conference Coverage

Navigating the Intersection of Radiation Therapy and Immunotherapy in Endometrial Cancer

As Orthopedic Oncology Evolves, Caring for the Clinician Must Be a Priority

Belumosudil Produces Long-Term Responses Without New Safety Concerns in cGVHD

Prophylactic Itacitinib May Safely Mitigate CRS Following Axi-Cel Administration in Lymphoma
2 Commerce Drive
Cranbury, NJ 08512