Using CRISPR to Learn How a Body Builds Itself
The popular gene-editing technique can deliver a step-by-step account of how a single-cell embryo becomes a trillion-cell animal.
Like every other animal, the nematode worm C. elegans begins life as a single fertilized cell. This divides into two, and then four, and then eight. By the time the worm is an adult, that original cell has become either 959 or 1031, depending on its sex. The number of cells never changes—it’s the same from one individual to another. And we know where every single one of them came from because a man named John Sulston spent 18 long months in the early 1980s, hand-drawing worms in a darkened room.
Sulston worked alone, in silence, hunched over a microscope for eight hours a day. By studying and drawing worms of various ages, he figured out the ancestor and descendants of each of their cells. It was a monumental piece of science. Sulston mapped the complete history of an individual, the comprehensive family tree of a single body. “We had the entire story of the worm’s cells from fertilized egg to adult,” he later said, upon accepting the Nobel Prize for his work.
Studying C. elegans was a smart call. The worm is small, so it only has a thousand or so cells. It’s transparent, so every cell could be easily observed. And it develops the same way every time, so each cell has an invariant fate. By contrast, mapping cell lineages in a bigger, opaque, and more variable animal—say, a fly or mouse—is much harder. Jay Shendure started thinking about ways of doing this in 2000, when he was a graduate student. “I worked for six months, and after my first lab meeting, a postdoc grabbed me. He said this would take a few decades to do,” Shendure recalls. “I switched projects but I always had this in the back of my head.”
Sixteen years on, Shendure is a professor at the University of Washington and he has finally created a way of mapping cell lineages in bigger animals. It works by creatively repurposing the most fashionable tool in modern biology—the gene-editing technique called CRISPR.
CRISPR involves two components—a scissor enzyme that slices DNA, and a guide molecule that can precisely deploy the scissors to the target of choice. Shendure’s technique involves inserting a consecutive row of said targets into a quiet corner of a cell’s DNA, creating a sequence that acts as a barcode. It gets sliced up, and when the cell repairs these cuts, it does so imperfectly, occasionally adding in extra letters or deleting existing ones.
When the cell divides, it passes its edited barcode on to its daughters, whose versions also get cut, repaired, and edited, and passed down in turn. On and on this goes. At every step, the barcode morphs a little bit more, randomly and irreversibly. Each cell inherits the cumulative tweaks of its ancestors, while developing its own unique changes which it passes on to its descendants. So you can reconstruct the history of the entire cell family by comparing their barcodes. What Sulston did by eye, Shendure’s team can do by sequencing.
They call the technique “genome editing of synthetic target arrays for lineage tracing” or GESTALT for short. “It’s a great starting point for understanding how a single cell gives rise to a complex animal, and a technique that dramatically improves on all prior attempts to do this,” says Leslie Vosshall from Rockefeller University.
For example, some scientists have labeled cells with dyes, while others have used viruses to smuggle foreign DNA into a cell’s genome. These techniques can tag all a cell’s descendants, but unlike GESTALT, those tags never change. They can tell you which cells belong to the same family, but not which descended from which. One way of doing that is to sequence the entire genomes of individual cells, to find and compare the mutations that they naturally accumulate. But that’s expensive, and impractical to do in bulk. With GESTALT, you only need to sequence the barcode. It’s considerably cheaper.
Team members Gregory Findlay and Aaron McKenna first proved that they could use GESTALT to trace lineages within groups of cells, growing in flasks. The next obvious move was to try it in C. elegans, to see if they could reproduce Sulston’s classic results. Instead, with what Vosshall calls “a great show of chutzpah,” they went straight to a far more complex animal—the zebrafish.
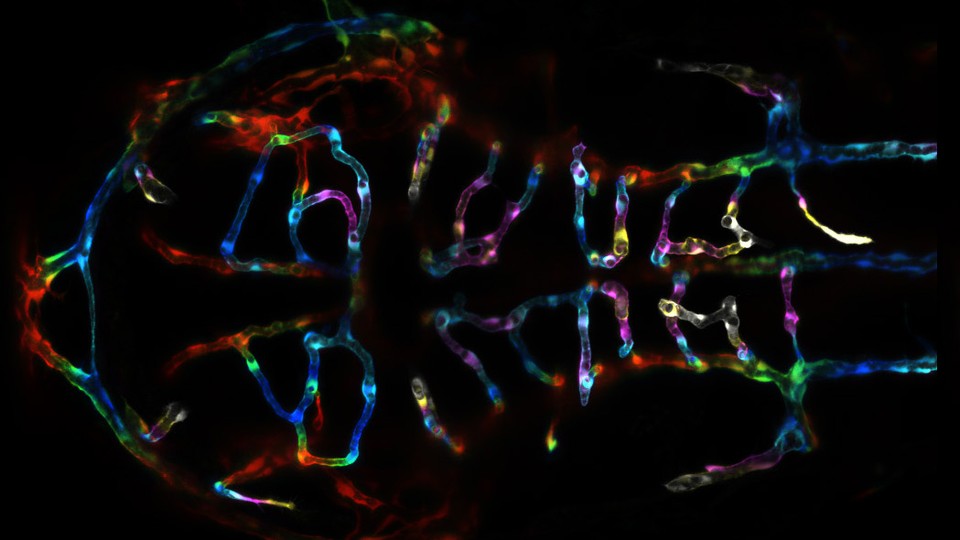
James Gagnon from Harvard University created a line of genetically engineered zebrafish carrying barcodes with ten CRISPR target sites. When the fish were just single-cell embryos, he injected them with the scissor enzyme and its guides. As the animals grew up, GESTALT went to work, producing thousands of unique barcodes. Gagnon could then read these in the adult fish.
In every organ he looked at, he found that most cells come from just a handful of progenitors. For example, just five ancestral cells gave rise to 98 percent of the red blood cells, and just 25 ancestors gave rise to more than 90 percent of the brain cells. Most of these progenitor cells were specific to their own organs; they carried barcodes that weren’t found elsewhere.
“It’s an ingenious application of CRISPR technology,” says Marnie Halpern from the Carnegie Institution for Science. Its biggest weakness, she says, is that it only reveals the lines of descent between a fish’s cells, without showing where those cells are in the animal. Marianne Bronner from the California Institute of Technology agrees. She suggests that the team could couple the barcodes with fluorescent molecules, so they could actually see the various lineages in a developing fish. Without that information, even GESTALT can’t produce the complete lineage trees that Sulston sketched out for C. elegans.
“This is the first iteration, and there are many ways of improving it to make it more powerful,” says Shendure. For now, the technique stalls early on in a fish’s life. The team want to tweak it so they can trace cell lineages well into adulthood. They also want to try GESTALT on other common laboratory animals, like flies and mice. It won’t work on humans because it’s absolutely forbidden to allow a genetically edited embryo to carry on to adulthood. But as Sulston showed, there’s a lot you can learn by unravelling the stories of supposedly simpler animals.
“There’s still an incredible amount to the development of complex organisms that we don’t understand,” says Shendure. “The fact that we found interesting things on this first pass is a sign of that.”
I’ve argued in the past that hyped and ethically fraught applications, like altering disease genes or making designer babies, distract from the true value of CRISPR. Its real power lies in allowing scientists to do the kinds of experiments that would have been utterly unfeasible just a few years ago. GESTALT is a perfect example.